Manganese »
PDB 4php-4qsf »
4qax »
Manganese in PDB 4qax: Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus
Enzymatic activity of Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus
All present enzymatic activity of Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus:
5.4.2.12;
5.4.2.12;
Protein crystallography data
The structure of Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus, PDB code: 4qax
was solved by
A.Roychowdhury,
A.Kundu,
M.Bose,
A.Gujar,
A.K.Das,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.53 / 2.00 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 72.802, 80.689, 89.254, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.6 / 21.4 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus
(pdb code 4qax). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus, PDB code: 4qax:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus, PDB code: 4qax:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 4qax
Go back to
Manganese binding site 1 out
of 2 in the Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus
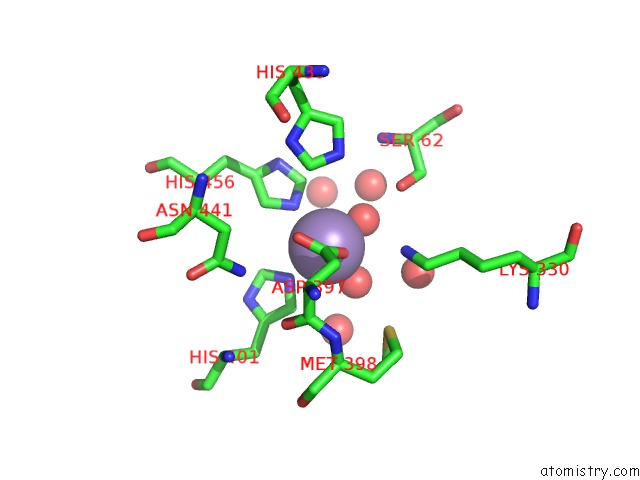
Mono view
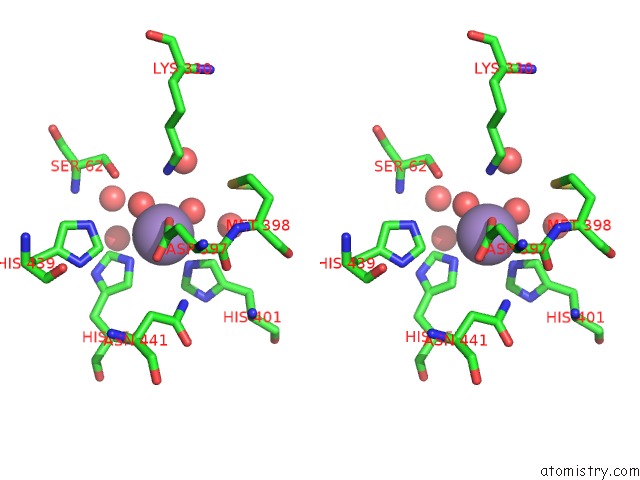
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus within 5.0Å range:
|
Manganese binding site 2 out of 2 in 4qax
Go back to
Manganese binding site 2 out
of 2 in the Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus
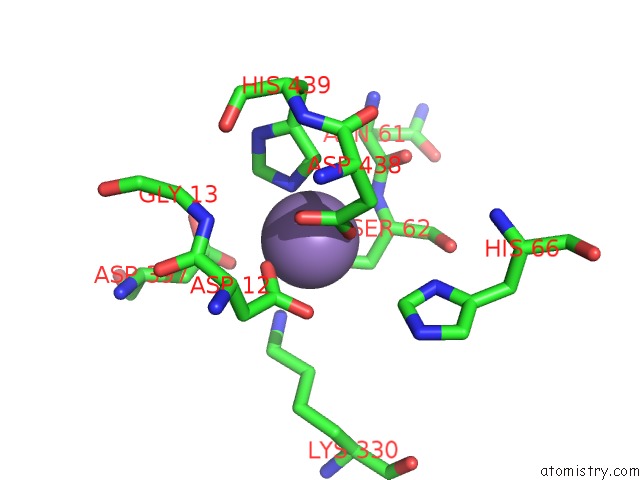
Mono view
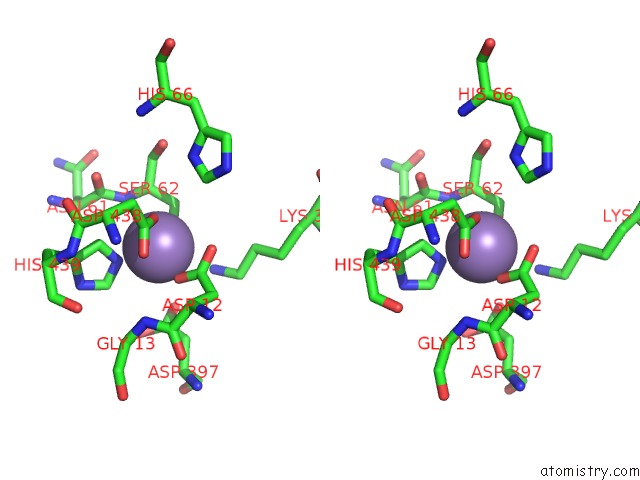
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Crystal Structure of Post-Catalytic Binary Complex of Phosphoglycerate Mutase From Staphylococcus Aureus within 5.0Å range:
|
Reference:
A.Roychowdhury,
A.Kundu,
M.Bose,
A.Gujar,
S.Mukherjee,
A.K.Das.
Structural and Funcctional Analysis of Phosphoglycerate Mutase From Staphylococcus Aureus To Be Published.
Page generated: Sat Oct 5 20:59:52 2024
Last articles
K in 1N0HK in 1N0X
K in 1MXC
K in 1MXB
K in 1MPW
K in 1MXA
K in 1MP0
K in 1MMF
K in 1MJI
K in 1MGY