Manganese »
PDB 4php-4qsf »
4pst »
Manganese in PDB 4pst: Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K
Protein crystallography data
The structure of Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K, PDB code: 4pst
was solved by
D.A.Keedy,
H.Van Den Bedem,
D.A.Sivak,
G.A.Petsko,
D.Ringe,
M.A.Wilson,
J.S.Fraser,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.40 / 1.05 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 34.299, 45.521, 98.711, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 12.1 / 14.2 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K
(pdb code 4pst). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K, PDB code: 4pst:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K, PDB code: 4pst:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 4pst
Go back to
Manganese binding site 1 out
of 2 in the Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K
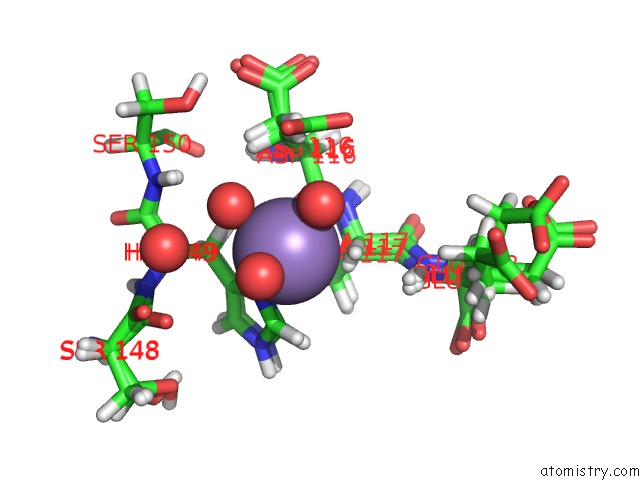
Mono view
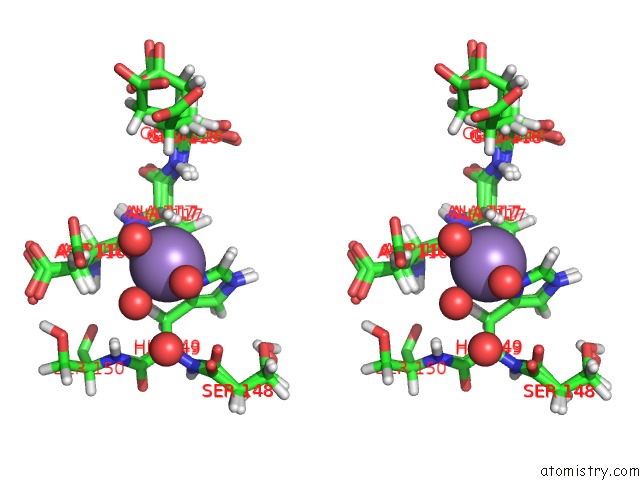
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K within 5.0Å range:
|
Manganese binding site 2 out of 2 in 4pst
Go back to
Manganese binding site 2 out
of 2 in the Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K
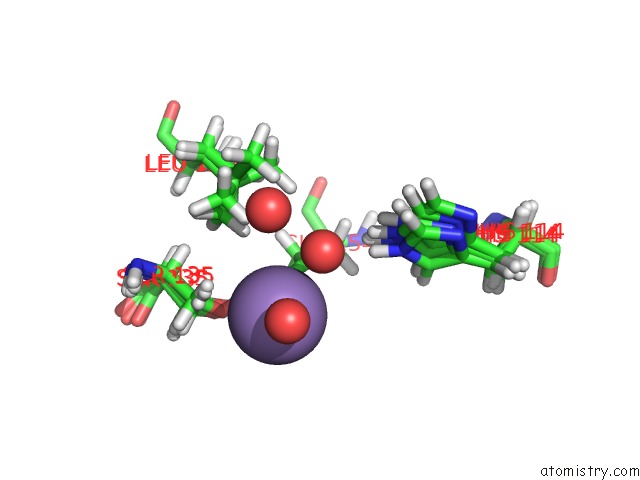
Mono view
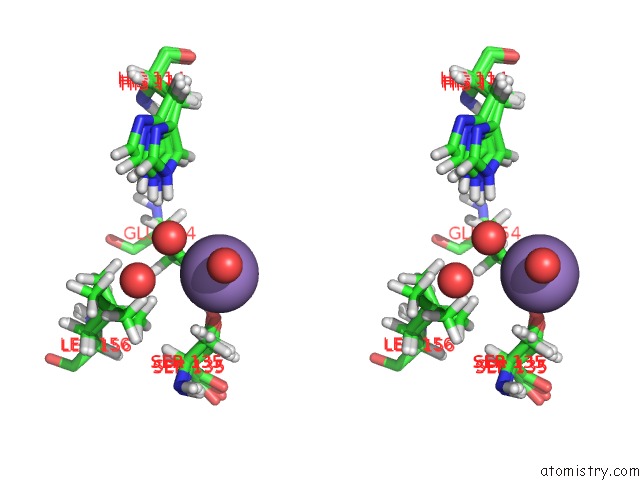
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Multiconformer Model For Escherichia Coli Dihydrofolate Reductase at 277 K within 5.0Å range:
|
Reference:
D.A.Keedy,
H.Van Den Bedem,
D.A.Sivak,
G.A.Petsko,
D.Ringe,
M.A.Wilson,
J.S.Fraser.
Crystal Cryocooling Distorts Conformational Heterogeneity in A Model Michaelis Complex of Dhfr. Structure V. 22 899 2014.
ISSN: ISSN 0969-2126
PubMed: 24882744
DOI: 10.1016/J.STR.2014.04.016
Page generated: Sat Oct 5 20:49:33 2024
ISSN: ISSN 0969-2126
PubMed: 24882744
DOI: 10.1016/J.STR.2014.04.016
Last articles
K in 1LJUK in 1LJL
K in 1LI9
K in 1L8I
K in 1LI0
K in 1LHR
K in 1L8H
K in 1KP8
K in 1L1H
K in 1L2X