Manganese »
PDB 4gwc-4ilk »
4hso »
Manganese in PDB 4hso: Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis
Enzymatic activity of Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis
All present enzymatic activity of Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis:
2.5.1.54;
2.5.1.54;
Protein crystallography data
The structure of Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis, PDB code: 4hso
was solved by
P.J.Cross,
A.L.Pietersma,
T.M.Allison,
S.M.Wilson-Coutts,
F.C.Cochrane,
E.J.Parker,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.53 / 2.10 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 73.523, 136.995, 76.212, 90.00, 96.63, 90.00 |
| R / Rfree (%) | 20.4 / 24.2 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis
(pdb code 4hso). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 4 binding sites of Manganese where determined in the Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis, PDB code: 4hso:
Jump to Manganese binding site number: 1; 2; 3; 4;
In total 4 binding sites of Manganese where determined in the Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis, PDB code: 4hso:
Jump to Manganese binding site number: 1; 2; 3; 4;
Manganese binding site 1 out of 4 in 4hso
Go back to
Manganese binding site 1 out
of 4 in the Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis
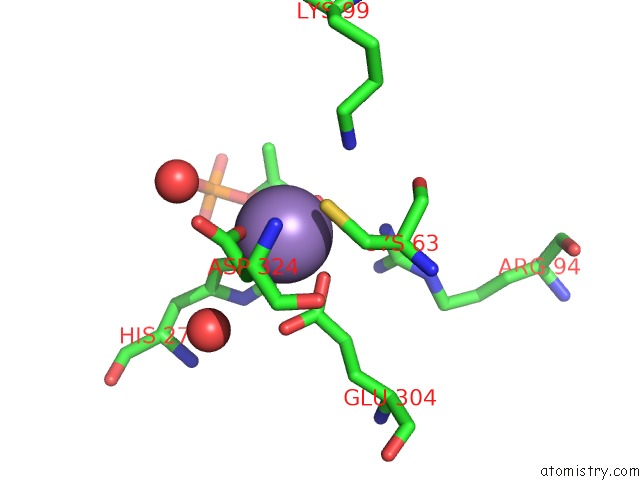
Mono view
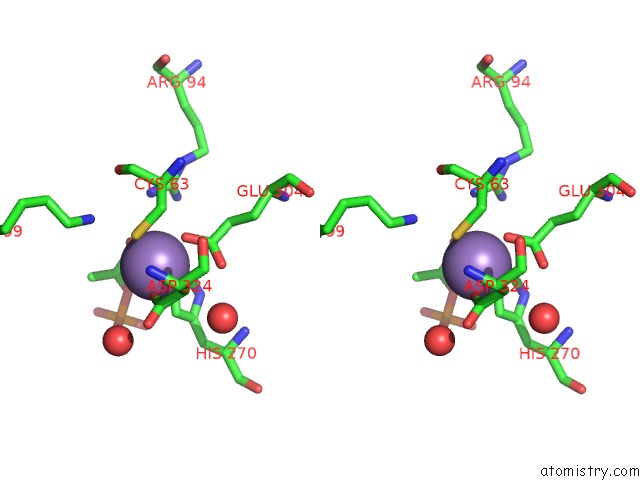
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis within 5.0Å range:
|
Manganese binding site 2 out of 4 in 4hso
Go back to
Manganese binding site 2 out
of 4 in the Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis
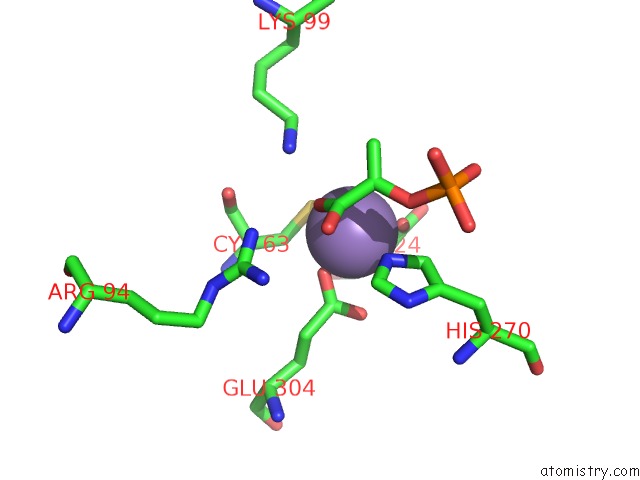
Mono view
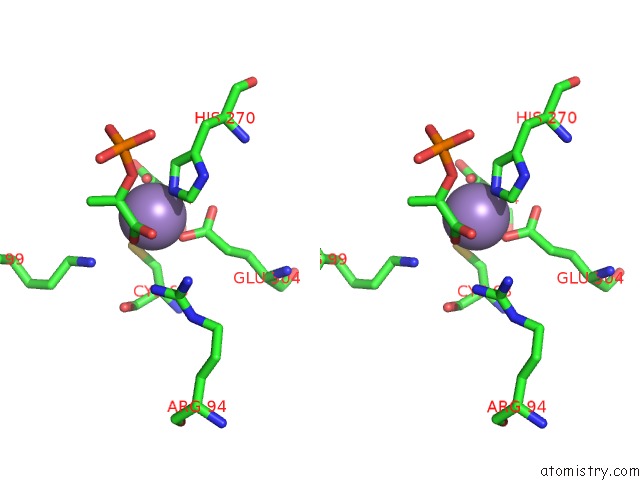
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis within 5.0Å range:
|
Manganese binding site 3 out of 4 in 4hso
Go back to
Manganese binding site 3 out
of 4 in the Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis
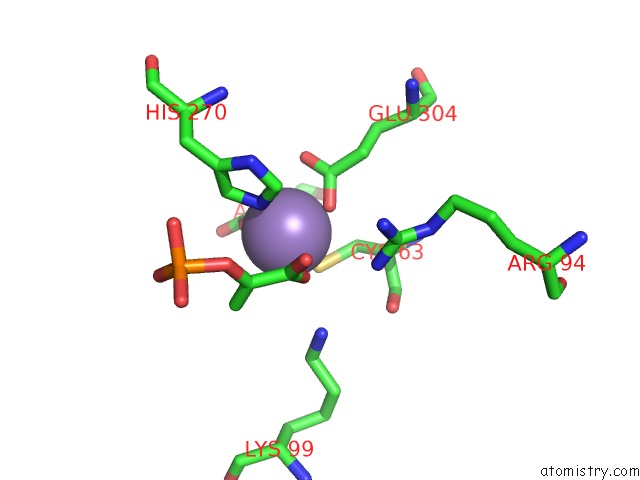
Mono view
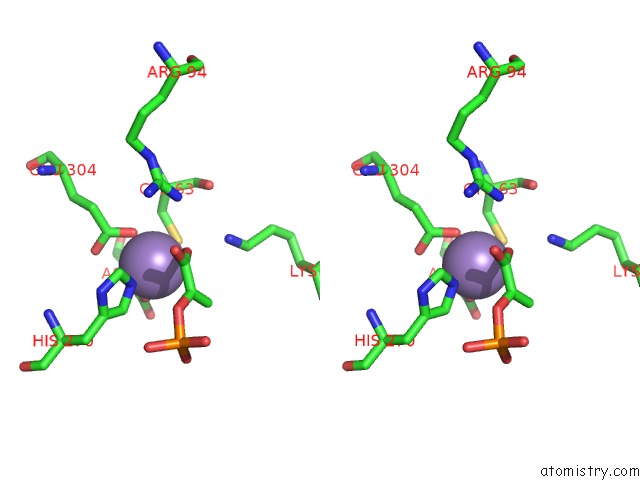
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis within 5.0Å range:
|
Manganese binding site 4 out of 4 in 4hso
Go back to
Manganese binding site 4 out
of 4 in the Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis
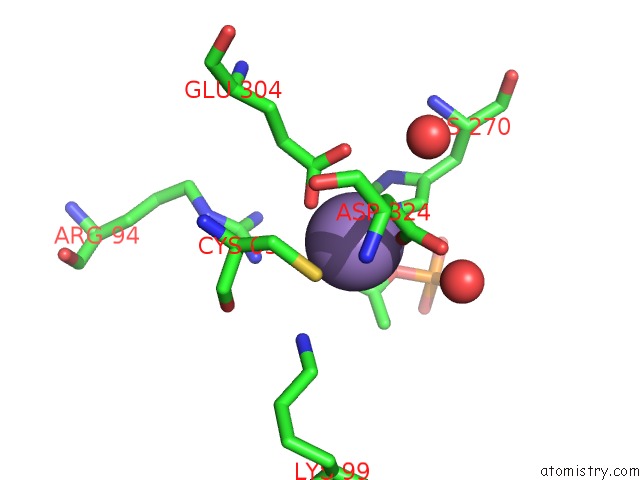
Mono view
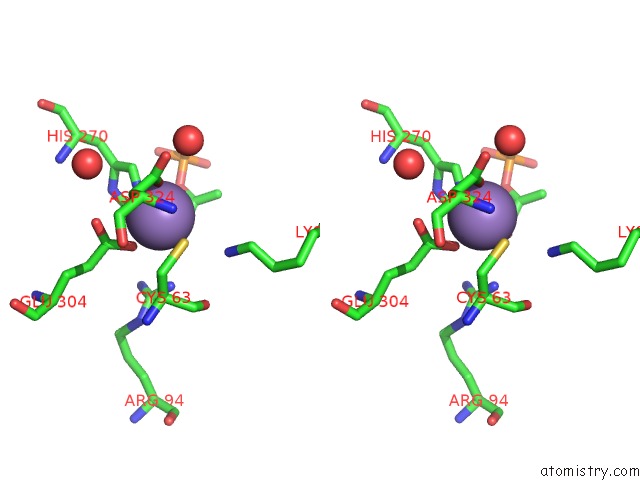
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of Crystal Structure of S213G Variant DAH7PS From Neisseria Meningitidis within 5.0Å range:
|
Reference:
P.J.Cross,
A.L.Pietersma,
T.M.Allison,
S.M.Wilson-Coutts,
F.C.Cochrane,
E.J.Parker.
Neisseria Meningitidis Expresses A Single 3-Deoxy-D-Arabino-Heptulosonate 7-Phosphate Synthase That Is Inhibited Primarily By Phenylalanine. Protein Sci. V. 22 1087 2013.
ISSN: ISSN 0961-8368
PubMed: 23754471
DOI: 10.1002/PRO.2293
Page generated: Sat Aug 16 14:13:01 2025
ISSN: ISSN 0961-8368
PubMed: 23754471
DOI: 10.1002/PRO.2293
Last articles
Mn in 4WIUMn in 4WIE
Mn in 4WFO
Mn in 4WFA
Mn in 4UXA
Mn in 4W8Y
Mn in 4W9S
Mn in 4V15
Mn in 4V0U
Mn in 4V0W