Manganese »
PDB 7mxs-7ohg »
7o5r »
Manganese in PDB 7o5r: Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1
Protein crystallography data
The structure of Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1, PDB code: 7o5r
was solved by
J.Laustsen,
I.Justo,
S.R.Marsden,
U.Hanefeld,
I.Bento,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 75.13 / 1.65 |
| Space group | H 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 71.54, 71.54, 225.394, 90, 90, 120 |
| R / Rfree (%) | 16 / 19.3 |
Other elements in 7o5r:
The structure of Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1 also contains other interesting chemical elements:
| Bromine | (Br) | 1 atom |
| Potassium | (K) | 3 atoms |
Manganese Binding Sites:
The binding sites of Manganese atom in the Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1
(pdb code 7o5r). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 3 binding sites of Manganese where determined in the Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1, PDB code: 7o5r:
Jump to Manganese binding site number: 1; 2; 3;
In total 3 binding sites of Manganese where determined in the Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1, PDB code: 7o5r:
Jump to Manganese binding site number: 1; 2; 3;
Manganese binding site 1 out of 3 in 7o5r
Go back to
Manganese binding site 1 out
of 3 in the Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1
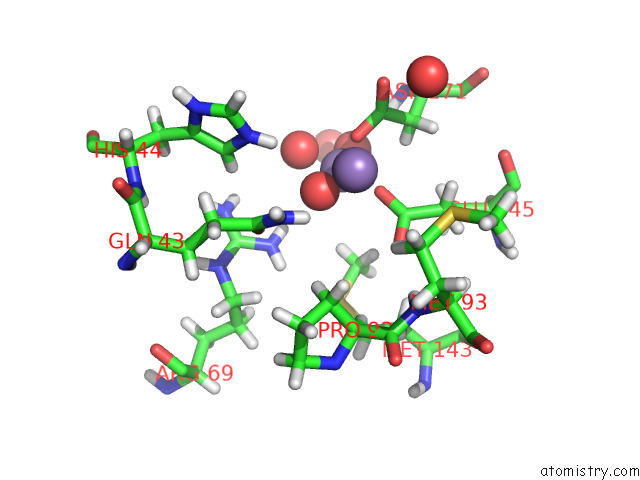
Mono view
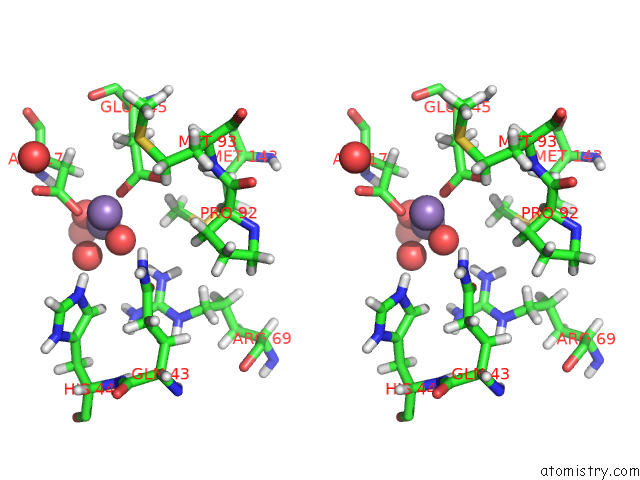
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1 within 5.0Å range:
|
Manganese binding site 2 out of 3 in 7o5r
Go back to
Manganese binding site 2 out
of 3 in the Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1
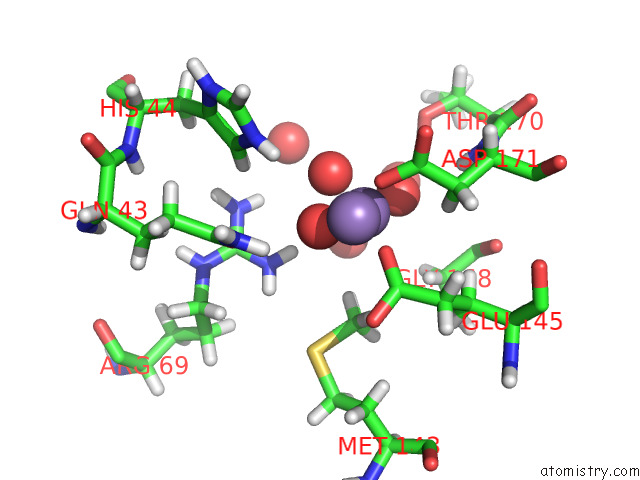
Mono view
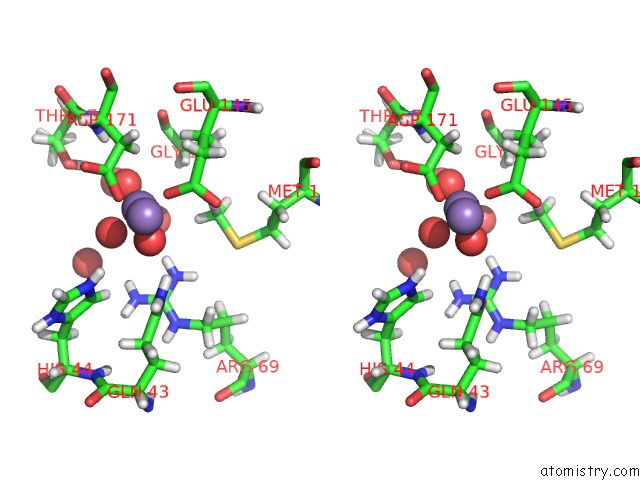
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1 within 5.0Å range:
|
Manganese binding site 3 out of 3 in 7o5r
Go back to
Manganese binding site 3 out
of 3 in the Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1
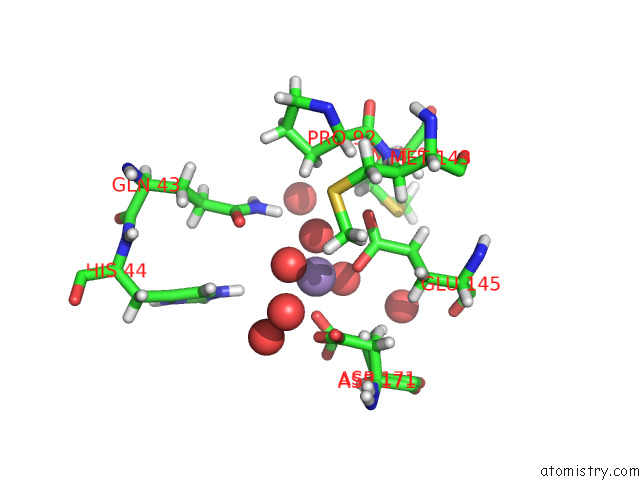
Mono view
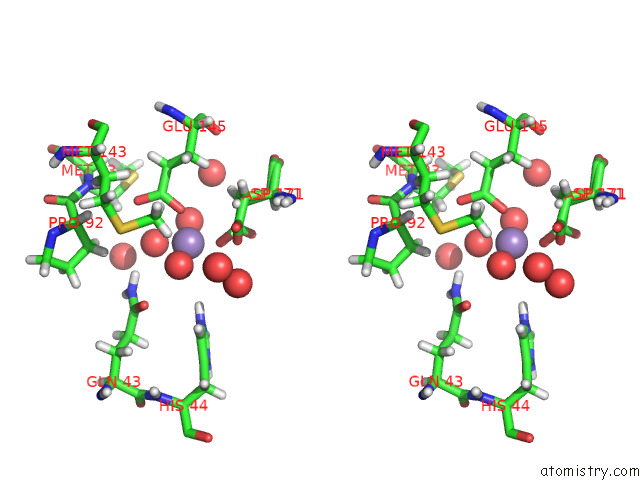
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Crystal Structure of Holo-Swhpa-Mn (Hydroxyketoacid Aldolase) From Sphingomonas Wittichii RW1 within 5.0Å range:
|
Reference:
S.R.Marsden,
H.J.Wijma,
M.K.F.Mohr,
I.Justo,
P.L.Hagedoorn,
J.Laustsen,
C.M.Jeffries,
D.Svergun,
L.Mestrom,
D.G.G.Mcmillan,
I.Bento,
U.Hanefeld.
Substrate Induced Movement of the Metal Cofactor Between Active and Resting State. Angew.Chem.Int.Ed.Engl. V. 61 13338 2022.
ISSN: ESSN 1521-3773
PubMed: 36214476
DOI: 10.1002/ANIE.202213338
Page generated: Sun Oct 6 10:18:45 2024
ISSN: ESSN 1521-3773
PubMed: 36214476
DOI: 10.1002/ANIE.202213338
Last articles
K in 9QDIK in 9R2L
K in 9QM5
K in 9QCG
K in 9OQE
K in 9OC4
K in 9OA8
K in 9O52
K in 9O7S
K in 9O51