Manganese »
PDB 6oe2-6q9f »
6pxu »
Manganese in PDB 6pxu: Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp
Enzymatic activity of Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp
All present enzymatic activity of Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp:
2.4.1.41;
2.4.1.41;
Protein crystallography data
The structure of Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp, PDB code: 6pxu
was solved by
N.L.Samara,
A.J.Fernandez,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.03 / 2.01 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 72.820, 73.123, 74.441, 113.08, 100.50, 108.23 |
| R / Rfree (%) | 17.1 / 21.9 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp
(pdb code 6pxu). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 4 binding sites of Manganese where determined in the Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp, PDB code: 6pxu:
Jump to Manganese binding site number: 1; 2; 3; 4;
In total 4 binding sites of Manganese where determined in the Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp, PDB code: 6pxu:
Jump to Manganese binding site number: 1; 2; 3; 4;
Manganese binding site 1 out of 4 in 6pxu
Go back to
Manganese binding site 1 out
of 4 in the Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp
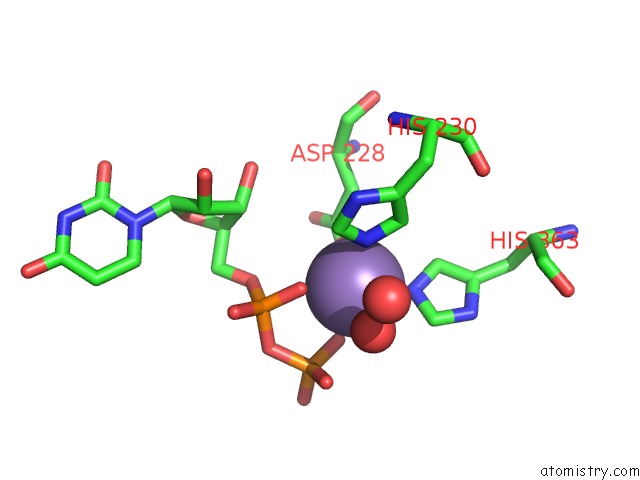
Mono view
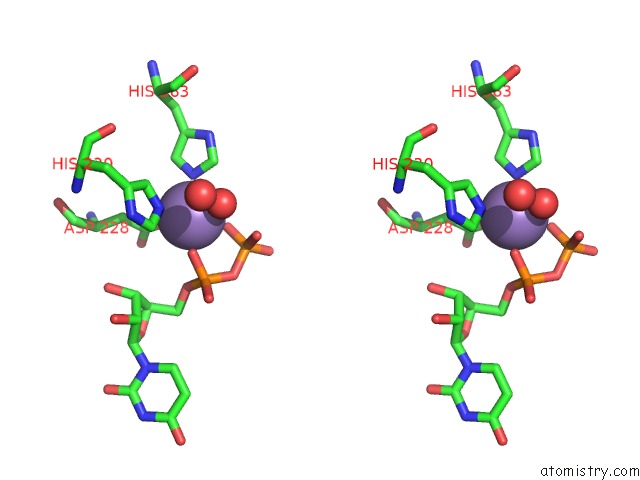
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp within 5.0Å range:
|
Manganese binding site 2 out of 4 in 6pxu
Go back to
Manganese binding site 2 out
of 4 in the Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp
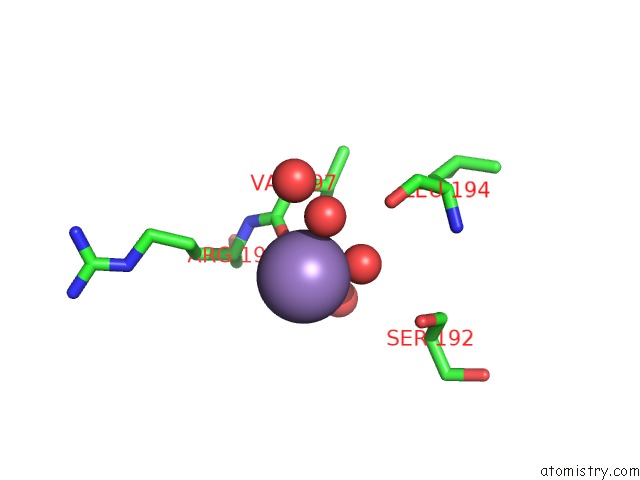
Mono view
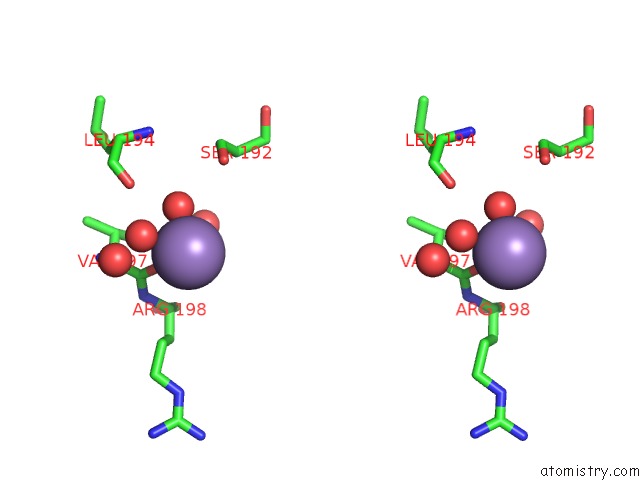
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp within 5.0Å range:
|
Manganese binding site 3 out of 4 in 6pxu
Go back to
Manganese binding site 3 out
of 4 in the Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp
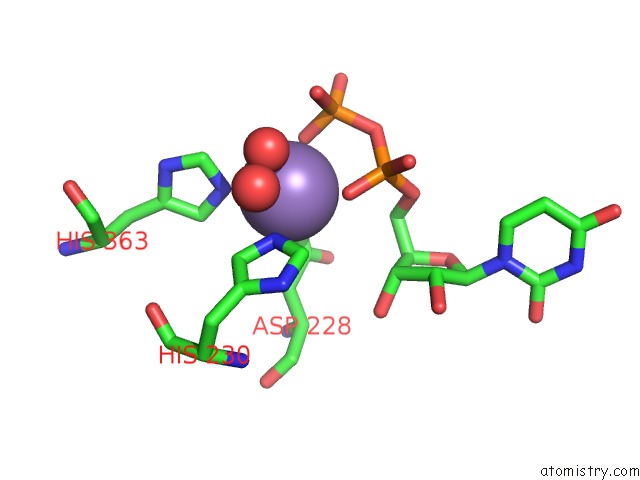
Mono view
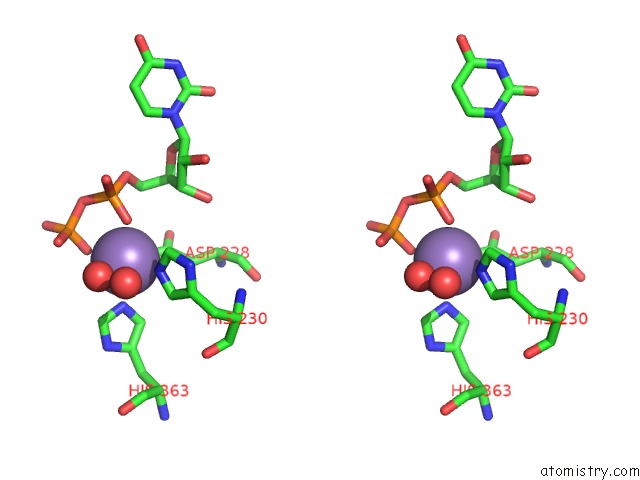
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp within 5.0Å range:
|
Manganese binding site 4 out of 4 in 6pxu
Go back to
Manganese binding site 4 out
of 4 in the Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp
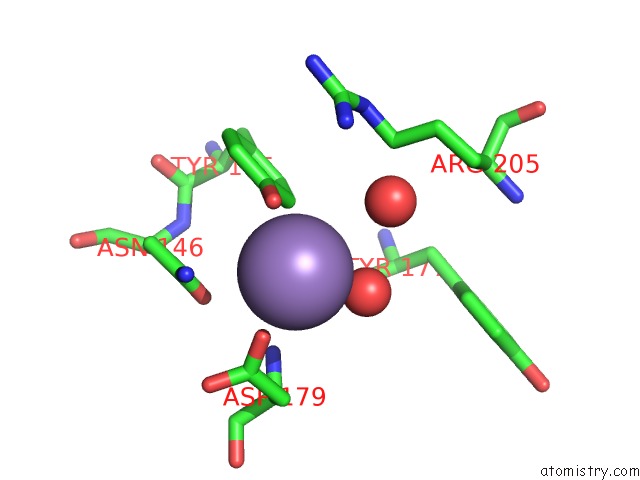
Mono view
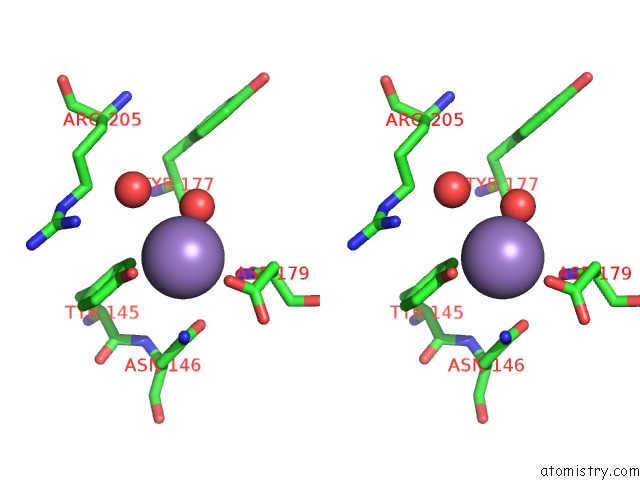
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of Crystal Structure of Human Galnac-T12 Bound to A Diglycosylated Peptide, MN2+, and Udp within 5.0Å range:
|
Reference:
A.J.Fernandez,
E.J.P.Daniel,
S.P.Mahajan,
J.J.Gray,
T.A.Gerken,
L.A.Tabak,
N.L.Samara.
The Structure of the Colorectal Cancer-Associated Enzyme Galnac-T12 Reveals How Nonconserved Residues Dictate Its Function. Proc.Natl.Acad.Sci.Usa V. 116 20404 2019.
ISSN: ESSN 1091-6490
PubMed: 31548401
DOI: 10.1073/PNAS.1902211116
Page generated: Sun Oct 6 05:52:42 2024
ISSN: ESSN 1091-6490
PubMed: 31548401
DOI: 10.1073/PNAS.1902211116
Last articles
K in 5CDBK in 5CCW
K in 5CE6
K in 5CCQ
K in 5CCR
K in 5CBW
K in 5CCN
K in 5CBV
K in 5C8Z
K in 5CBT