Manganese »
PDB 4z8b-5a56 »
4za5 »
Manganese in PDB 4za5: Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms.
Enzymatic activity of Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms.
All present enzymatic activity of Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms.:
4.1.1.61;
4.1.1.61;
Protein crystallography data
The structure of Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms., PDB code: 4za5
was solved by
K.A.P.Payne,
D.Leys,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.01 / 1.10 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 96.020, 63.790, 87.720, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14.4 / 15.6 |
Other elements in 4za5:
The structure of Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms. also contains other interesting chemical elements:
| Potassium | (K) | 3 atoms |
Manganese Binding Sites:
The binding sites of Manganese atom in the Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms.
(pdb code 4za5). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms., PDB code: 4za5:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms., PDB code: 4za5:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 4za5
Go back to
Manganese binding site 1 out
of 2 in the Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms.
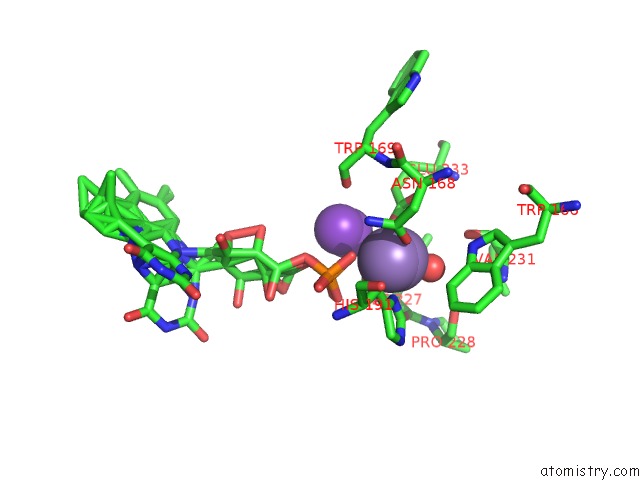
Mono view
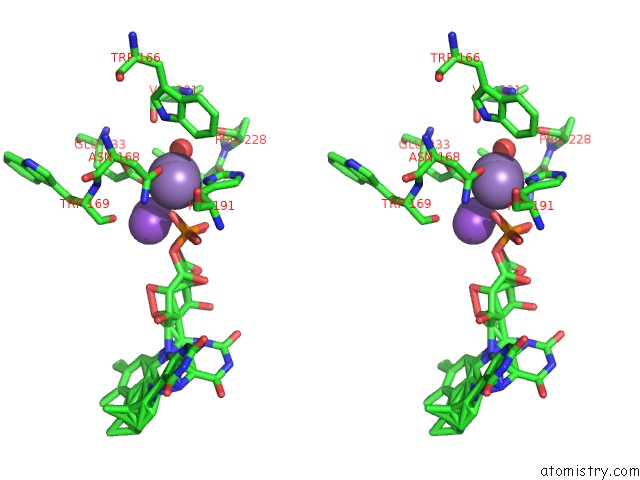
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms. within 5.0Å range:
|
Manganese binding site 2 out of 2 in 4za5
Go back to
Manganese binding site 2 out
of 2 in the Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms.
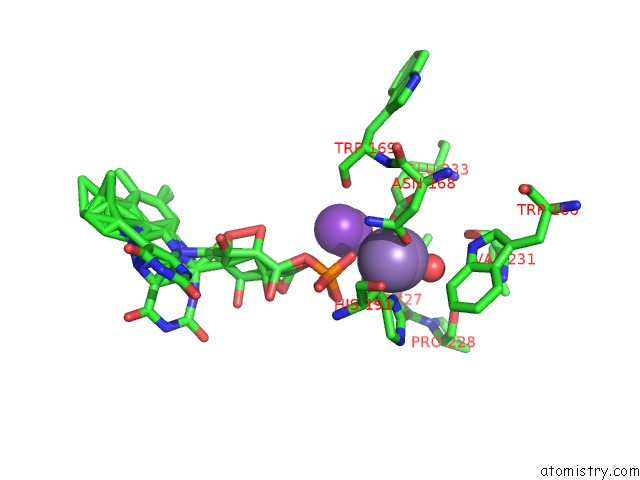
Mono view
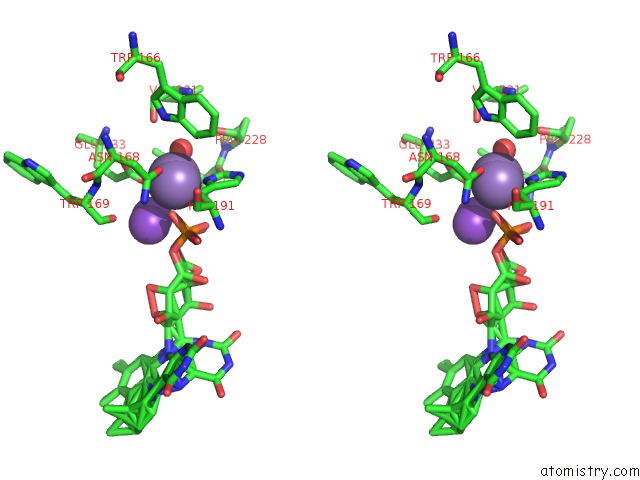
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Structure of A. Niger FDC1 with the Prenylated-Flavin Cofactor in the Iminium and Ketimine Forms. within 5.0Å range:
|
Reference:
K.A.Payne,
M.D.White,
K.Fisher,
B.Khara,
S.S.Bailey,
D.Parker,
N.J.Rattray,
D.K.Trivedi,
R.Goodacre,
R.Beveridge,
P.Barran,
S.E.Rigby,
N.S.Scrutton,
S.Hay,
D.Leys.
New Cofactor Supports Alpha , Beta-Unsaturated Acid Decarboxylation Via 1,3-Dipolar Cycloaddition. Nature V. 522 502 2015.
ISSN: ESSN 1476-4687
PubMed: 26083754
DOI: 10.1038/NATURE14560
Page generated: Sat Oct 5 23:12:11 2024
ISSN: ESSN 1476-4687
PubMed: 26083754
DOI: 10.1038/NATURE14560
Last articles
Mg in 5IVGMg in 5IUL
Mg in 5IUM
Mg in 5IRP
Mg in 5IUK
Mg in 5IUJ
Mg in 5IUC
Mg in 5IU0
Mg in 5IT5
Mg in 5ITZ