Manganese »
PDB 2v3z-2wgz »
2vqa »
Manganese in PDB 2vqa: Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca.
Protein crystallography data
The structure of Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca., PDB code: 2vqa
was solved by
S.Tottey,
K.J.Waldron,
S.J.Firbank,
B.Reale,
C.Bessant,
K.Sato,
J.Gray,
M.J.Banfield,
C.Dennison,
N.J.Robinson,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 58.32 / 2.95 |
| Space group | P 65 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 236.190, 236.190, 134.041, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 19.1 / 23.3 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca.
(pdb code 2vqa). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 6 binding sites of Manganese where determined in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca., PDB code: 2vqa:
Jump to Manganese binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Manganese where determined in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca., PDB code: 2vqa:
Jump to Manganese binding site number: 1; 2; 3; 4; 5; 6;
Manganese binding site 1 out of 6 in 2vqa
Go back to
Manganese binding site 1 out
of 6 in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca.
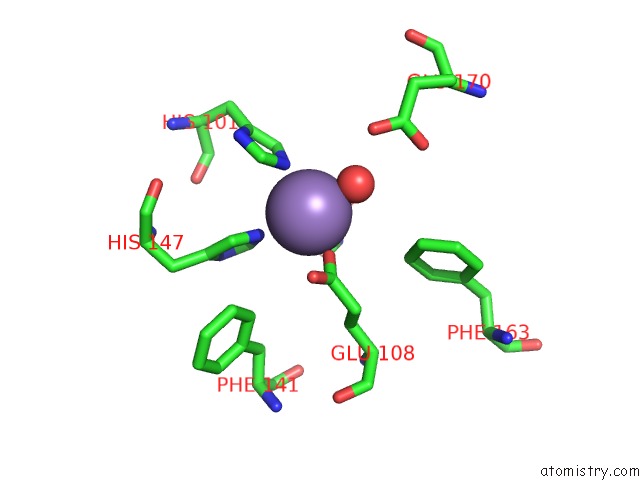
Mono view
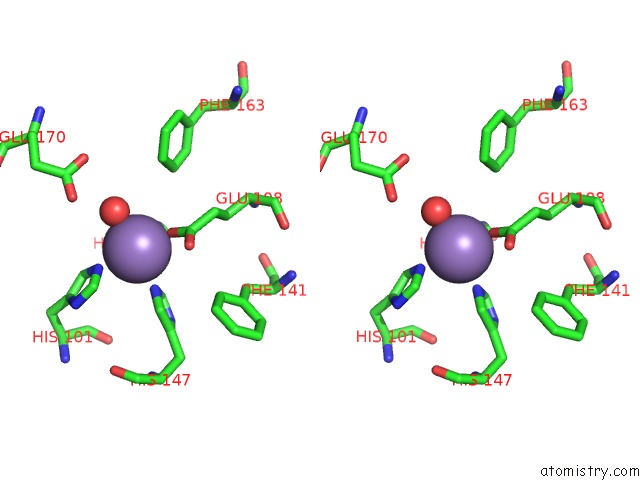
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca. within 5.0Å range:
|
Manganese binding site 2 out of 6 in 2vqa
Go back to
Manganese binding site 2 out
of 6 in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca.
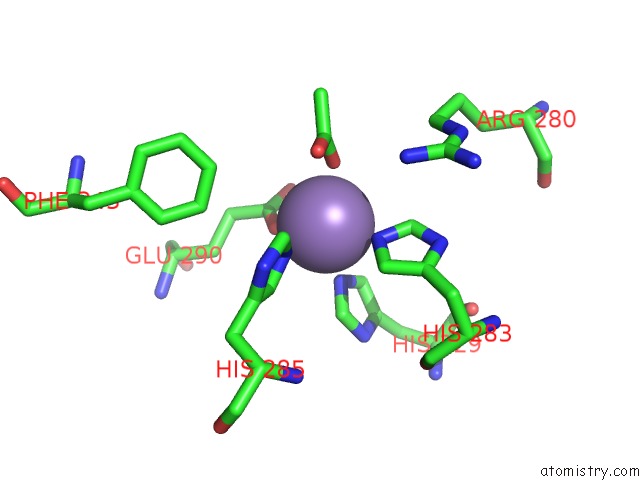
Mono view
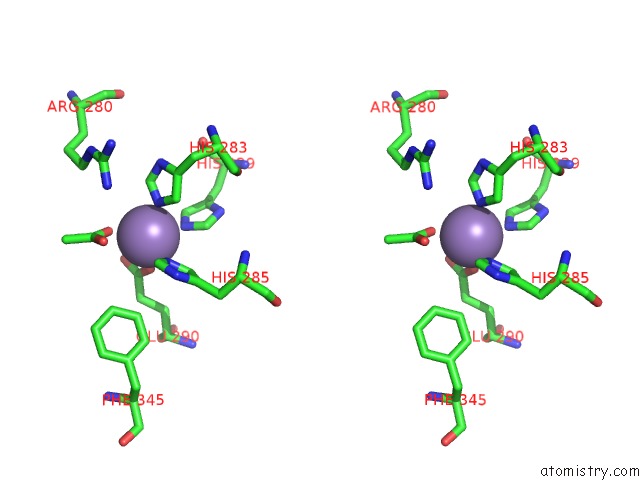
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca. within 5.0Å range:
|
Manganese binding site 3 out of 6 in 2vqa
Go back to
Manganese binding site 3 out
of 6 in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca.
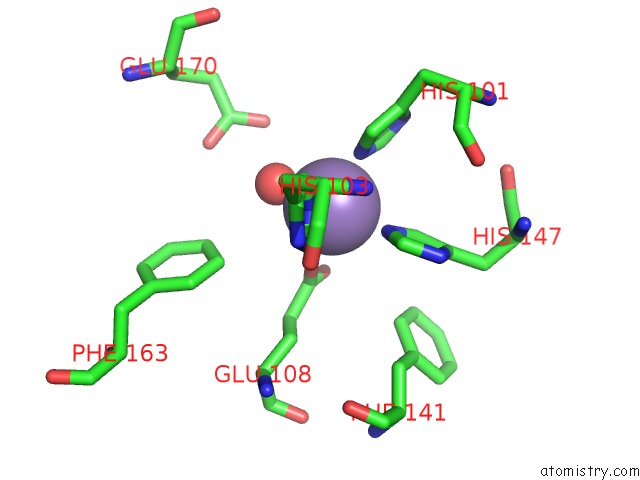
Mono view
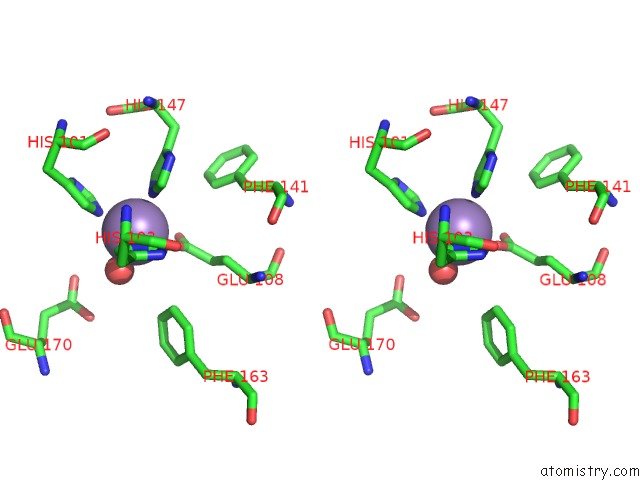
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca. within 5.0Å range:
|
Manganese binding site 4 out of 6 in 2vqa
Go back to
Manganese binding site 4 out
of 6 in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca.
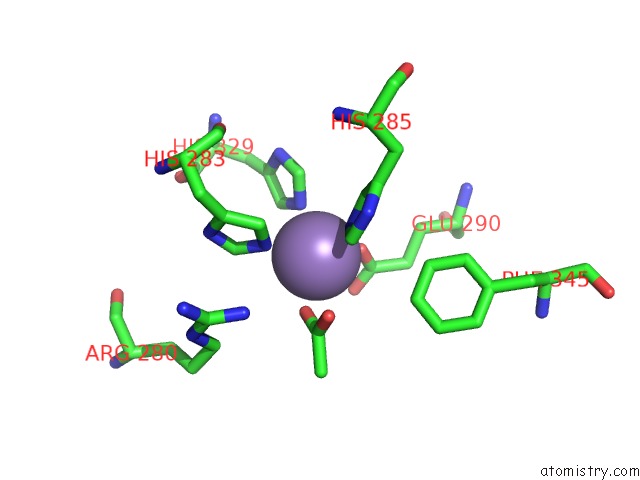
Mono view
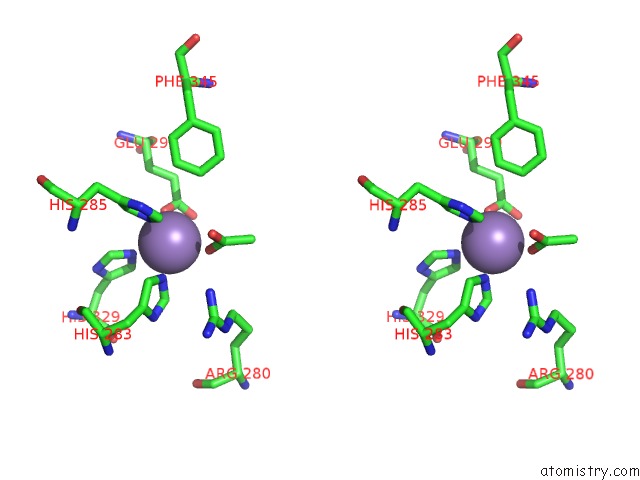
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca. within 5.0Å range:
|
Manganese binding site 5 out of 6 in 2vqa
Go back to
Manganese binding site 5 out
of 6 in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca.
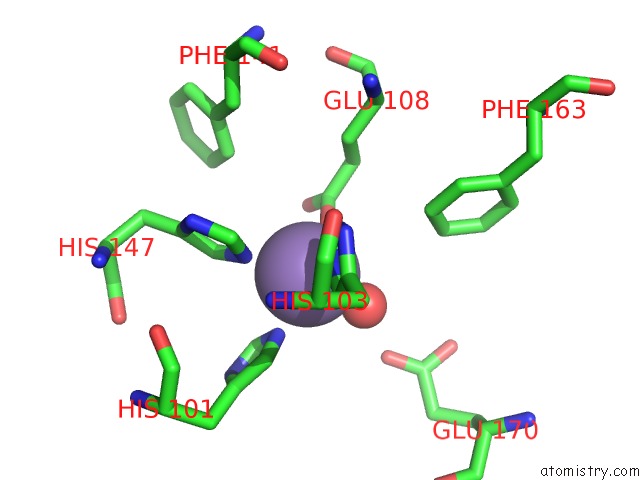
Mono view
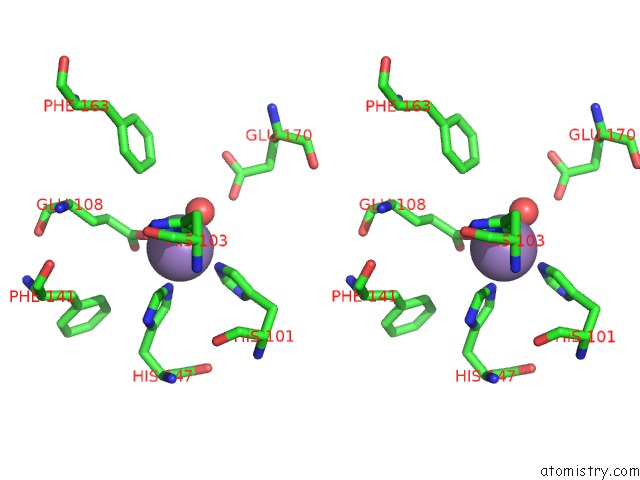
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 5 of Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca. within 5.0Å range:
|
Manganese binding site 6 out of 6 in 2vqa
Go back to
Manganese binding site 6 out
of 6 in the Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca.
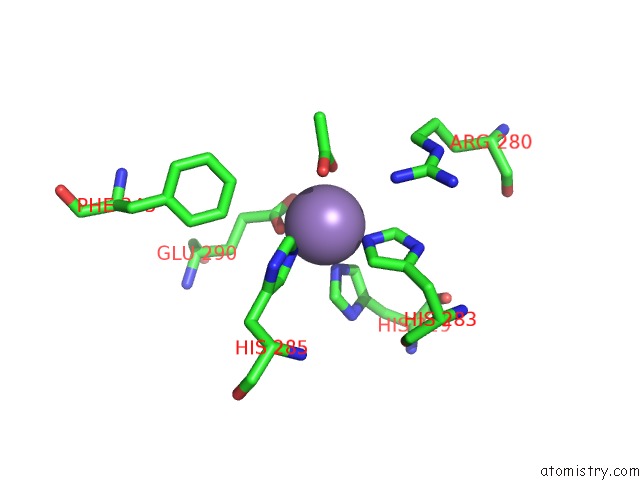
Mono view
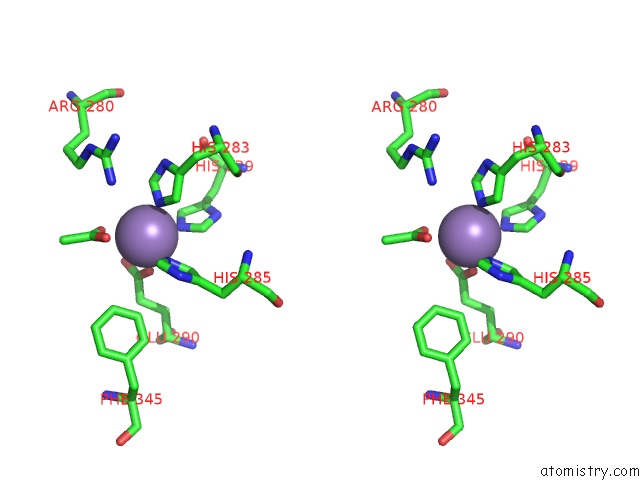
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 6 of Protein-Folding Location Can Regulate Mn Versus Cu- or Zn- Binding. Crystal Structure of Mnca. within 5.0Å range:
|
Reference:
S.Tottey,
K.J.Waldron,
S.J.Firbank,
B.Reale,
C.Bessant,
K.Sato,
T.R.Cheek,
J.Gray,
M.J.Banfield,
C.Dennison,
N.J.Robinson.
Protein-Folding Location Can Regulate Manganese-Binding Versus Copper- or Zinc-Binding. Nature V. 455 1138 2008.
ISSN: ISSN 0028-0836
PubMed: 18948958
DOI: 10.1038/NATURE07340
Page generated: Sat Oct 5 15:18:37 2024
ISSN: ISSN 0028-0836
PubMed: 18948958
DOI: 10.1038/NATURE07340
Last articles
K in 2YHKK in 2YCP
K in 2YGH
K in 2YD0
K in 2YFY
K in 2YCT
K in 2YCN
K in 2YCB
K in 2XTL
K in 2Y4O