Manganese »
PDB 8q3z-8slm »
8ro4 »
Manganese in PDB 8ro4: The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens
Protein crystallography data
The structure of The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens, PDB code: 8ro4
was solved by
C.Grininger,
J.Bitter,
M.Pfeiffer,
B.Nidetzky,
T.Pavkov-Keller,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.04 / 2.51 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 82.32, 165.55, 93.38, 90, 113.34, 90 |
| R / Rfree (%) | 18.1 / 21.7 |
Manganese Binding Sites:
The binding sites of Manganese atom in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens
(pdb code 8ro4). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 6 binding sites of Manganese where determined in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens, PDB code: 8ro4:
Jump to Manganese binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Manganese where determined in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens, PDB code: 8ro4:
Jump to Manganese binding site number: 1; 2; 3; 4; 5; 6;
Manganese binding site 1 out of 6 in 8ro4
Go back to
Manganese binding site 1 out
of 6 in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens
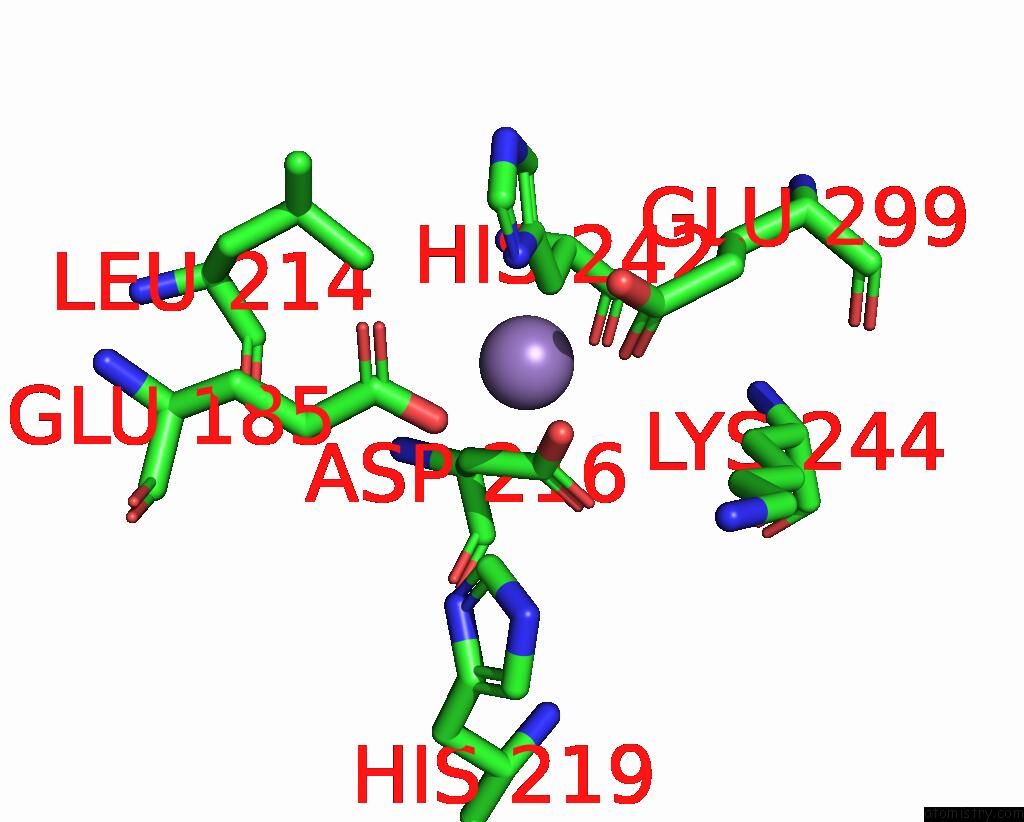
Mono view
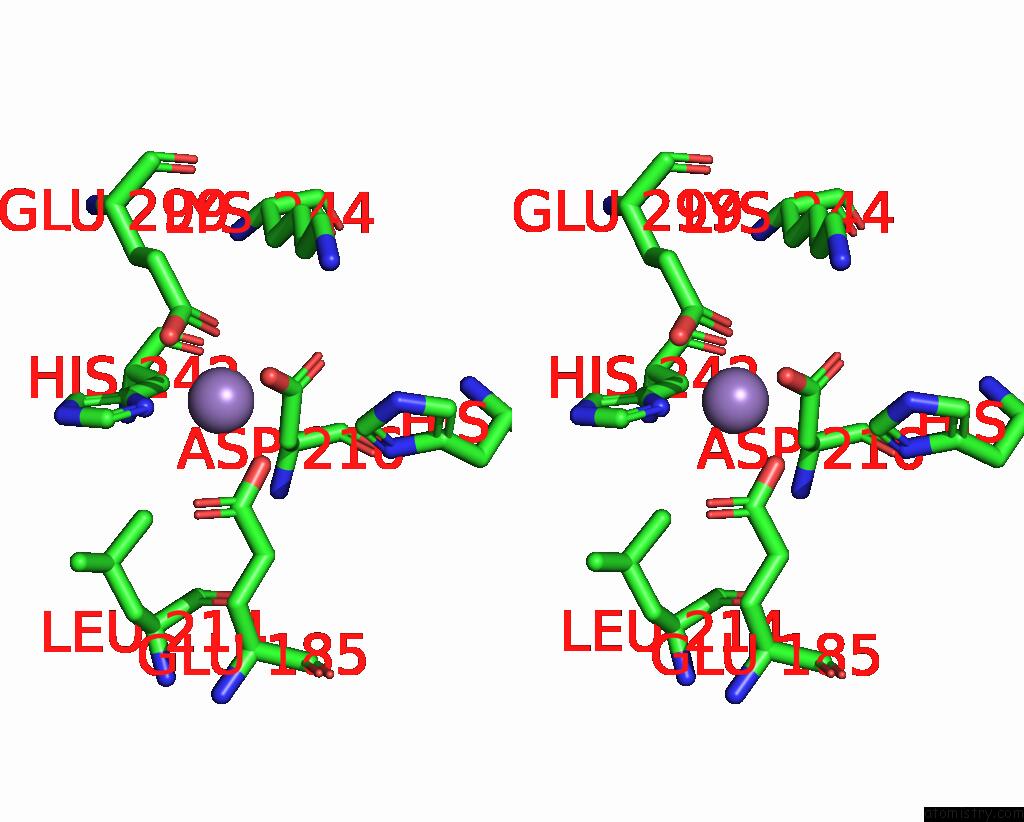
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens within 5.0Å range:
|
Manganese binding site 2 out of 6 in 8ro4
Go back to
Manganese binding site 2 out
of 6 in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens
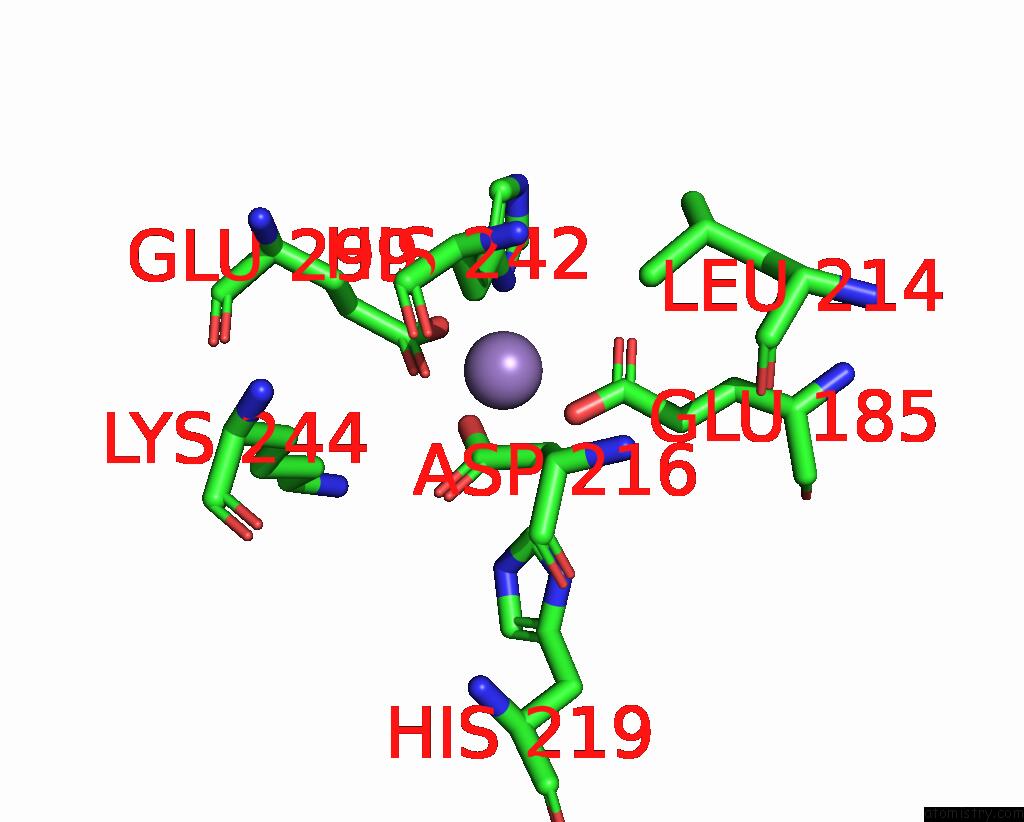
Mono view
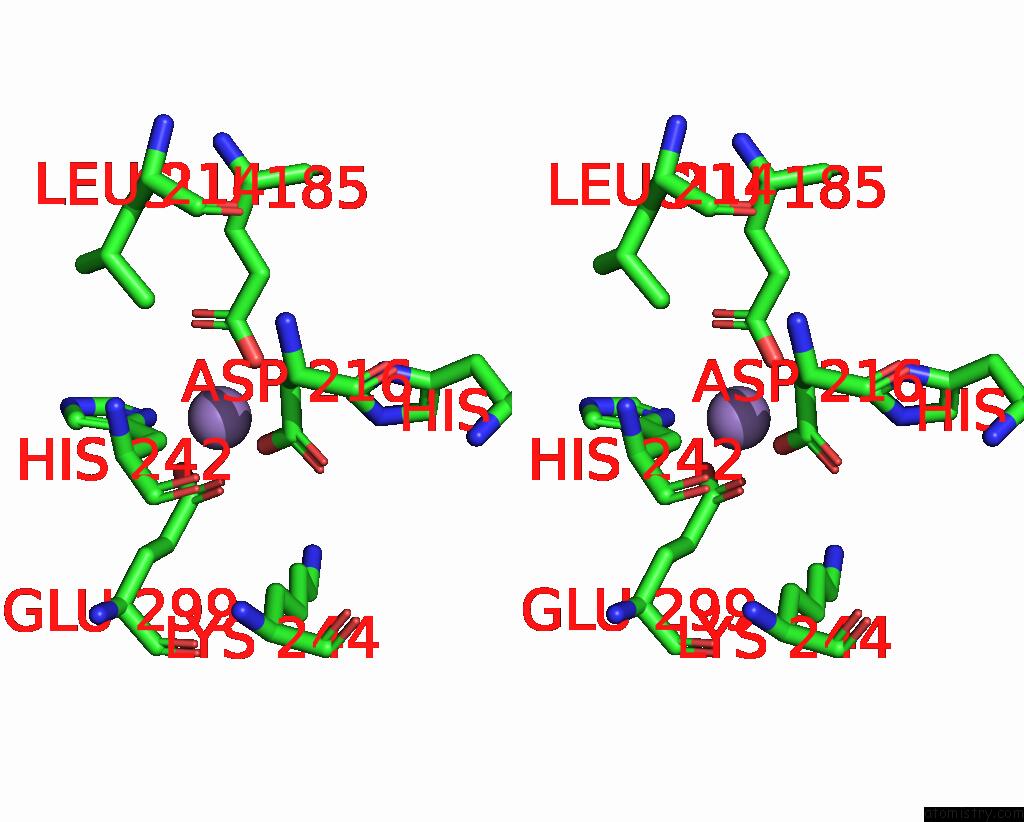
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens within 5.0Å range:
|
Manganese binding site 3 out of 6 in 8ro4
Go back to
Manganese binding site 3 out
of 6 in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens
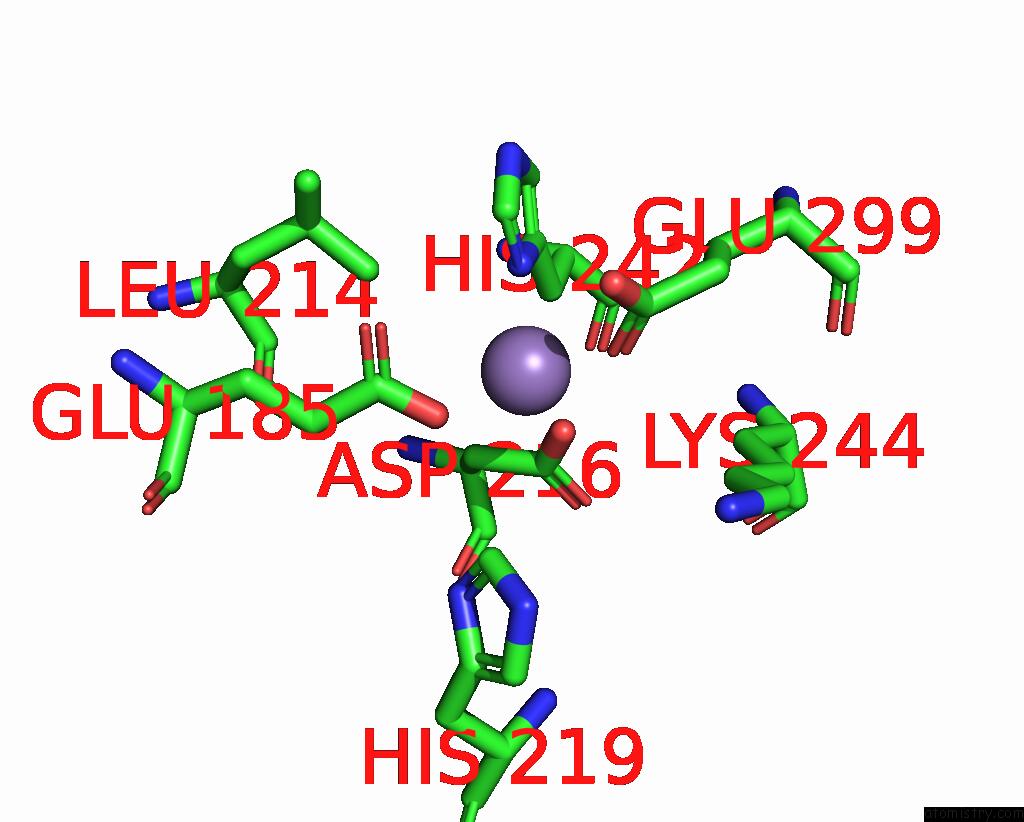
Mono view
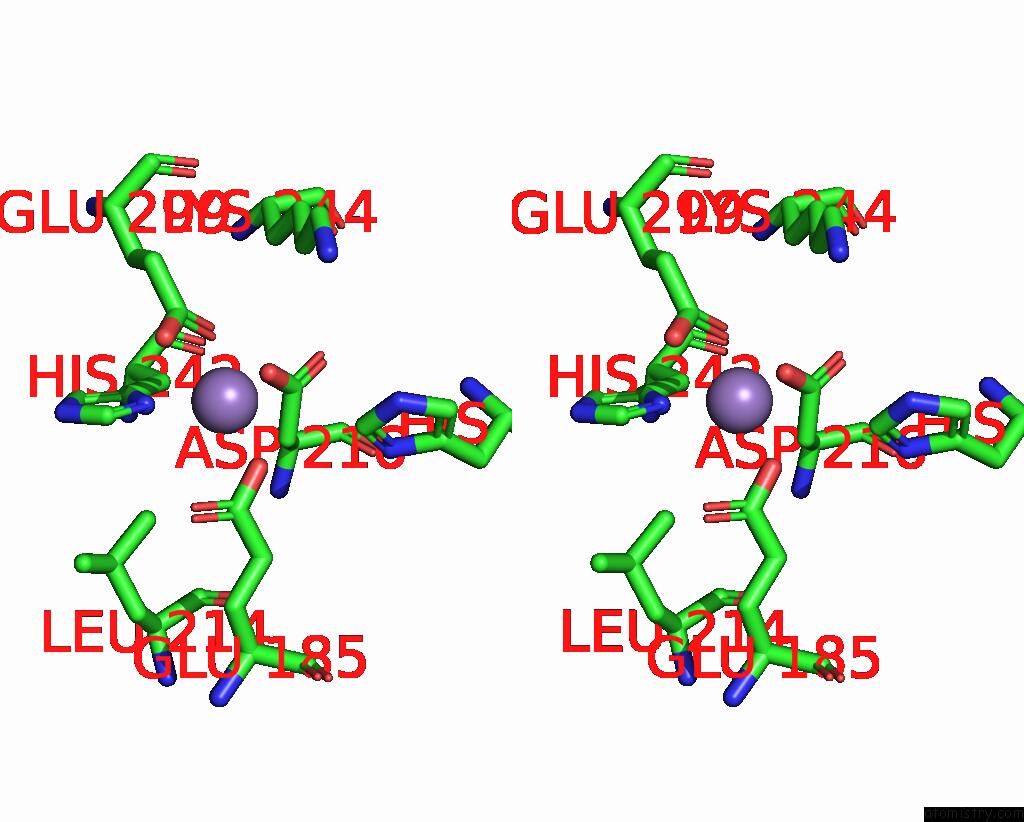
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens within 5.0Å range:
|
Manganese binding site 4 out of 6 in 8ro4
Go back to
Manganese binding site 4 out
of 6 in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens
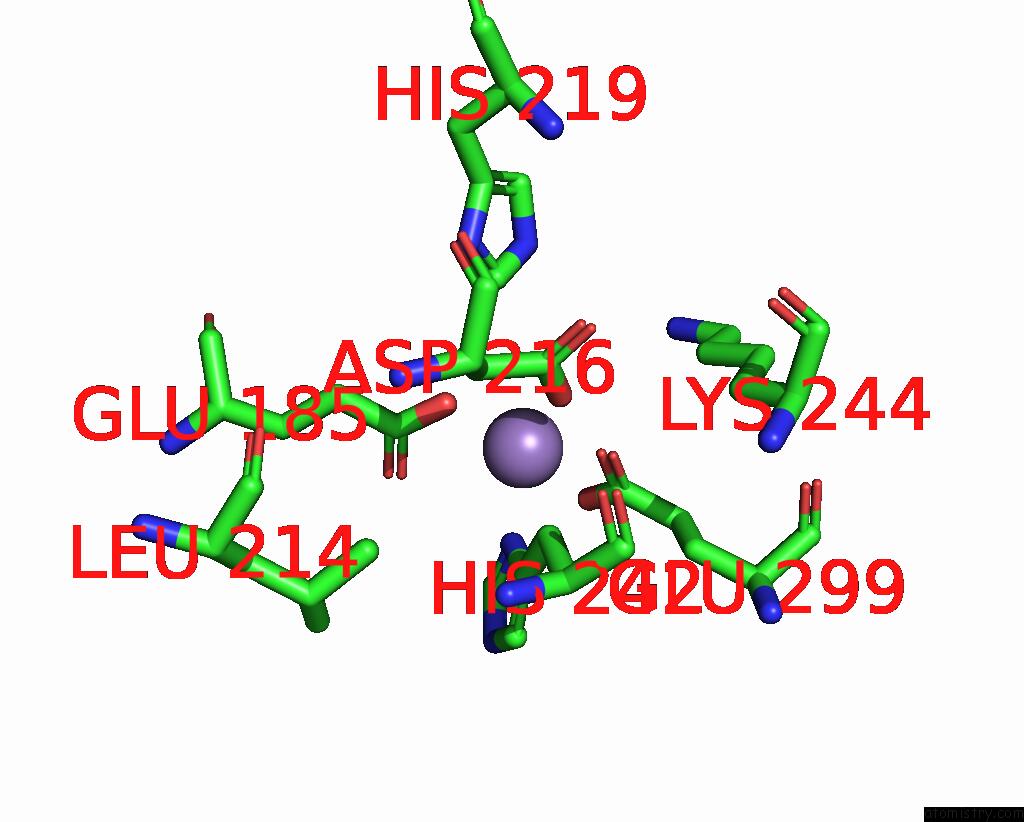
Mono view
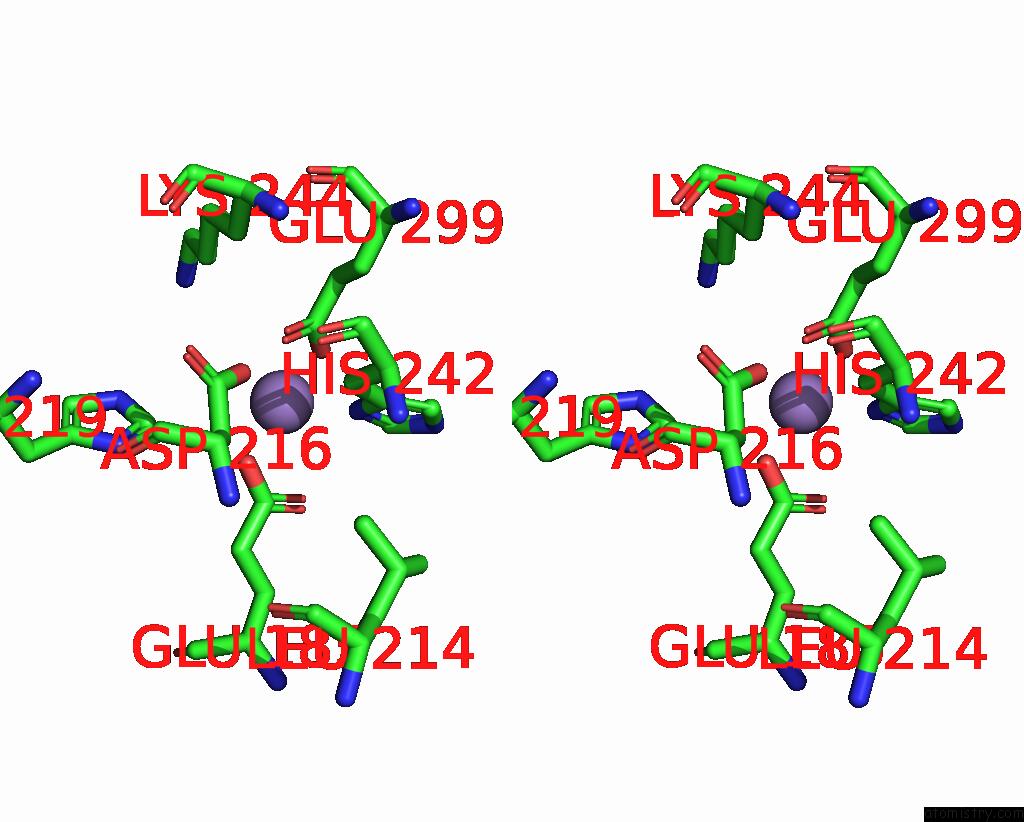
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens within 5.0Å range:
|
Manganese binding site 5 out of 6 in 8ro4
Go back to
Manganese binding site 5 out
of 6 in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens
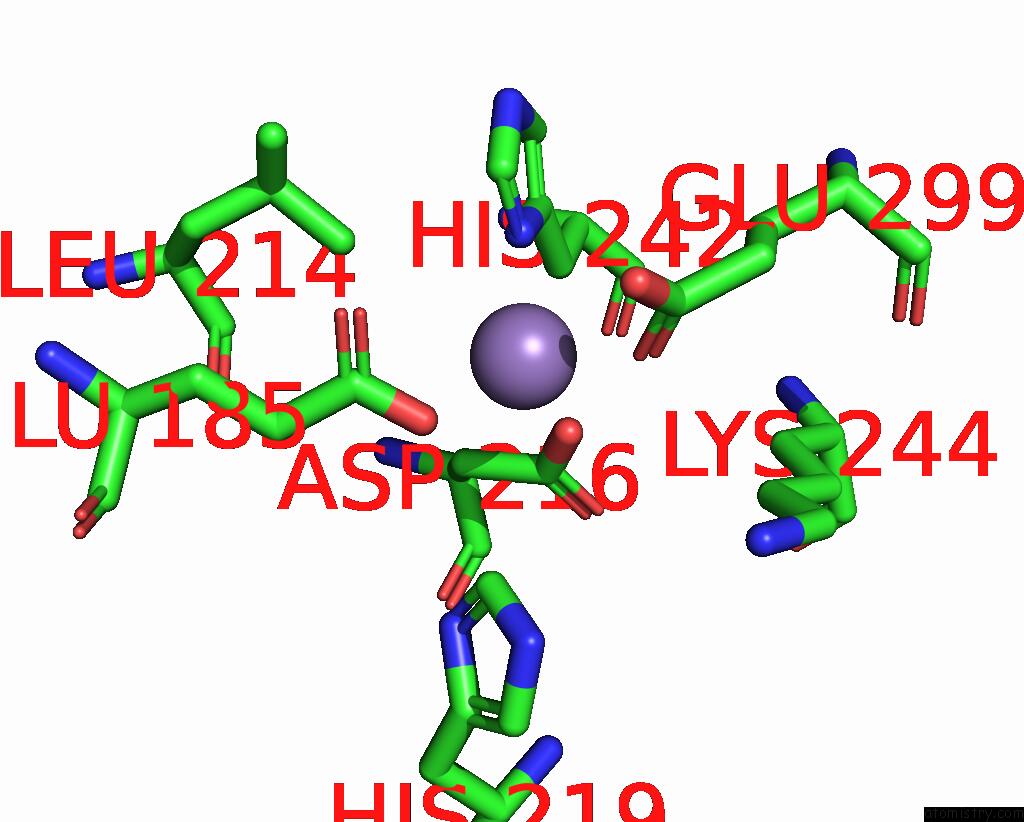
Mono view
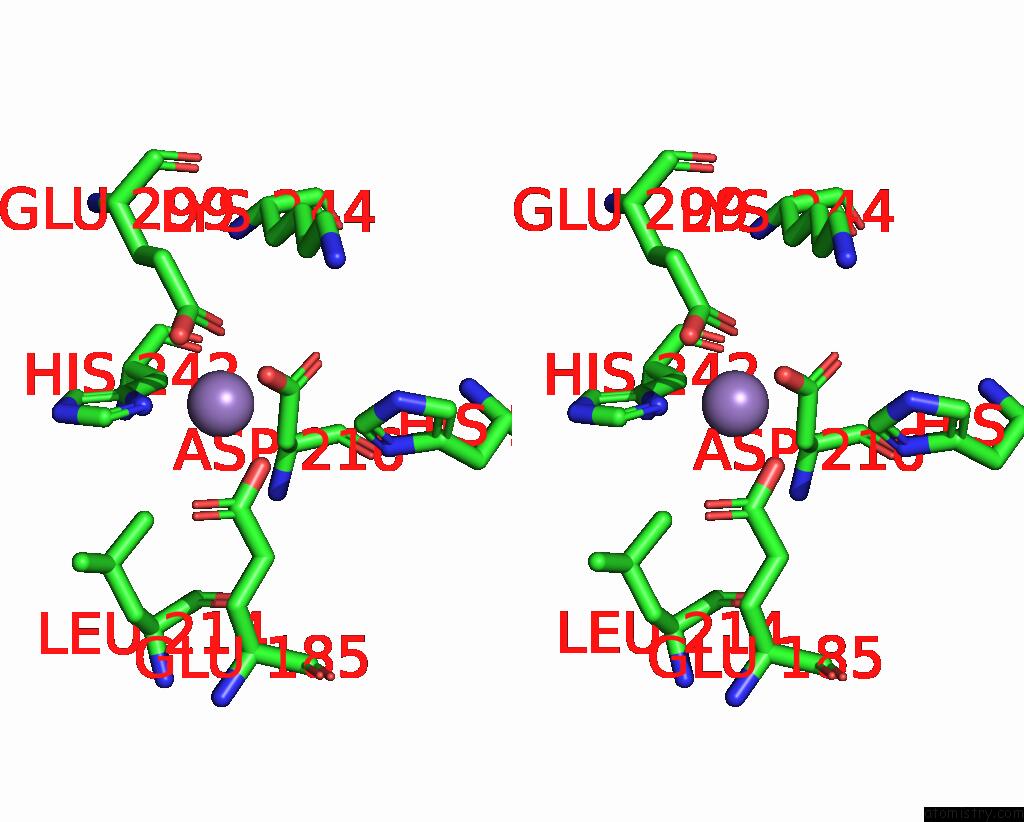
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 5 of The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens within 5.0Å range:
|
Manganese binding site 6 out of 6 in 8ro4
Go back to
Manganese binding site 6 out
of 6 in the The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens
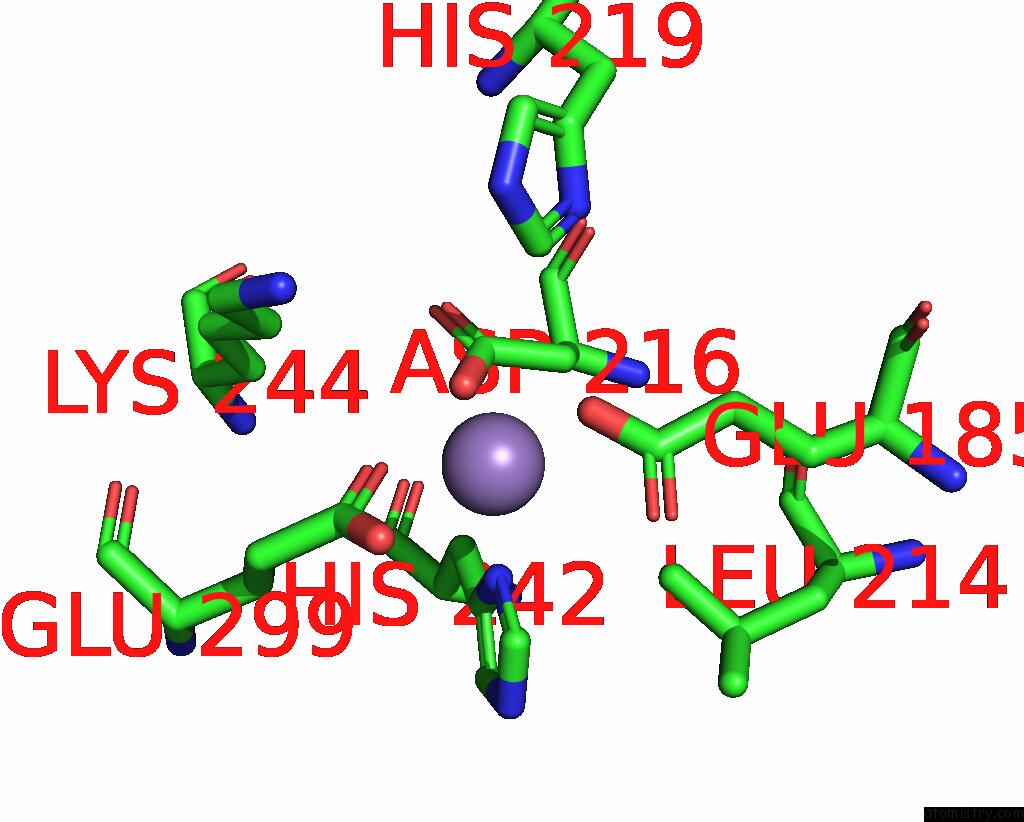
Mono view
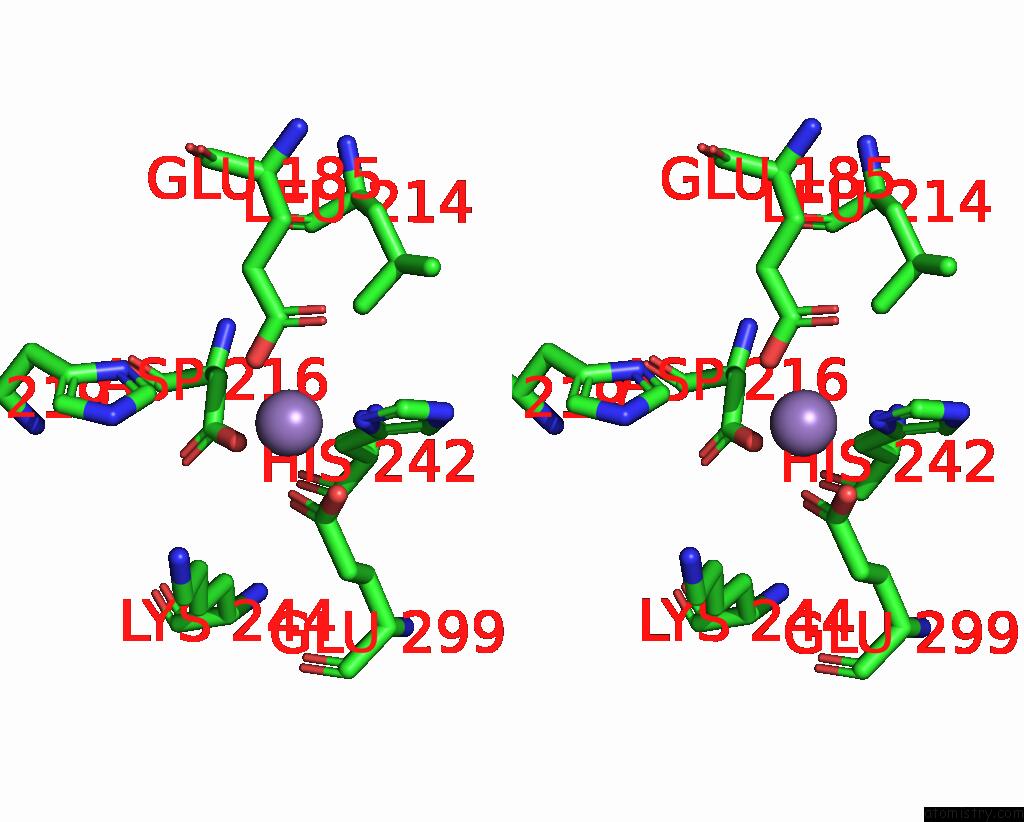
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 6 of The Crystal Structure of 2-Hydroxy-3-Keto-Glucal Hydratase Athyd From A. Tumefaciens within 5.0Å range:
|
Reference:
K.Kastner,
J.Bitter,
M.Pfeiffer,
C.Grininger,
G.Oberdorfer,
T.Pavkov-Keller,
H.Weber,
B.Nidetzky.
Enzyme Machinery For Bacterial Glucoside Metabolism Through A Conserved Non-Hydrolytic Pathway. Angew.Chem.Int.Ed.Engl. 10681 2024.
ISSN: ESSN 1521-3773
PubMed: 39041709
DOI: 10.1002/ANIE.202410681
Page generated: Sun Oct 6 13:47:08 2024
ISSN: ESSN 1521-3773
PubMed: 39041709
DOI: 10.1002/ANIE.202410681
Last articles
Cl in 7U0ACl in 7TZX
Cl in 7TZW
Cl in 7TZY
Cl in 7TXR
Cl in 7TZ6
Cl in 7TZN
Cl in 7TZM
Cl in 7TXN
Cl in 7TXO