Manganese »
PDB 8hlf-8irh »
8i8t »
Manganese in PDB 8i8t: Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+
Enzymatic activity of Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+
All present enzymatic activity of Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+:
3.6.1.55; 3.6.1.56;
3.6.1.55; 3.6.1.56;
Protein crystallography data
The structure of Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+, PDB code: 8i8t
was solved by
T.Nakamura,
Y.Yamagata,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 44.37 / 1.22 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 46.476, 47.512, 124.061, 90, 90, 90 |
| R / Rfree (%) | 15 / 18 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+
(pdb code 8i8t). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 4 binding sites of Manganese where determined in the Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+, PDB code: 8i8t:
Jump to Manganese binding site number: 1; 2; 3; 4;
In total 4 binding sites of Manganese where determined in the Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+, PDB code: 8i8t:
Jump to Manganese binding site number: 1; 2; 3; 4;
Manganese binding site 1 out of 4 in 8i8t
Go back to
Manganese binding site 1 out
of 4 in the Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+
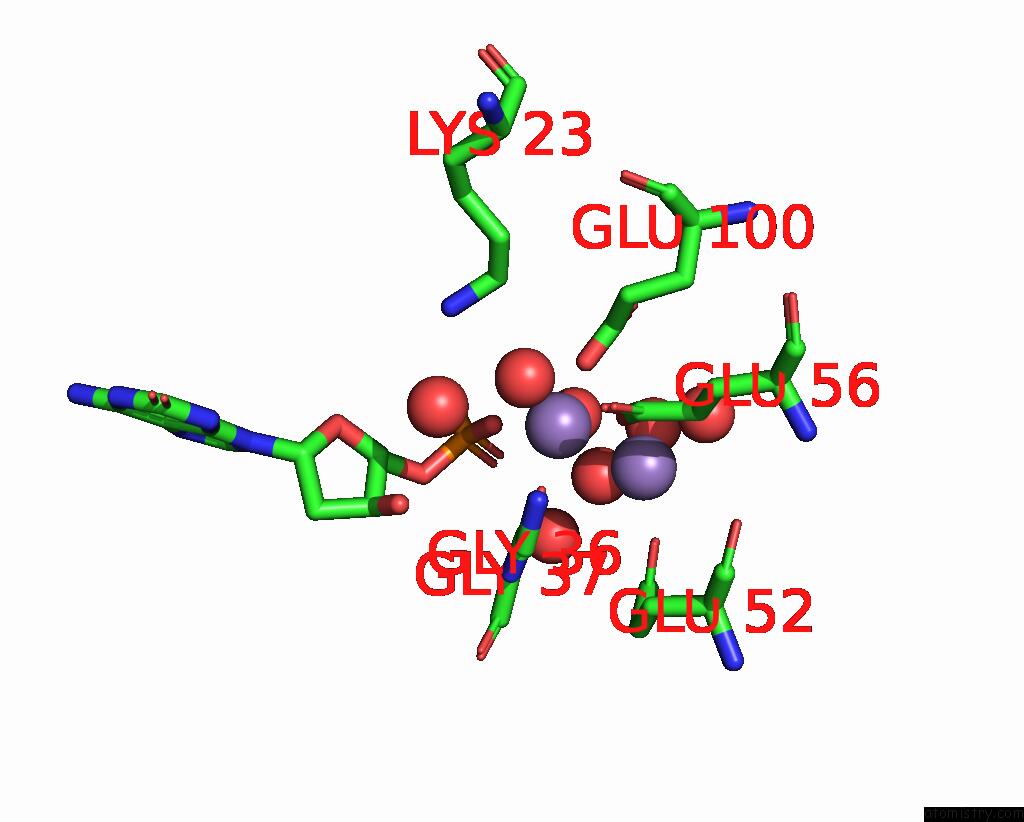
Mono view
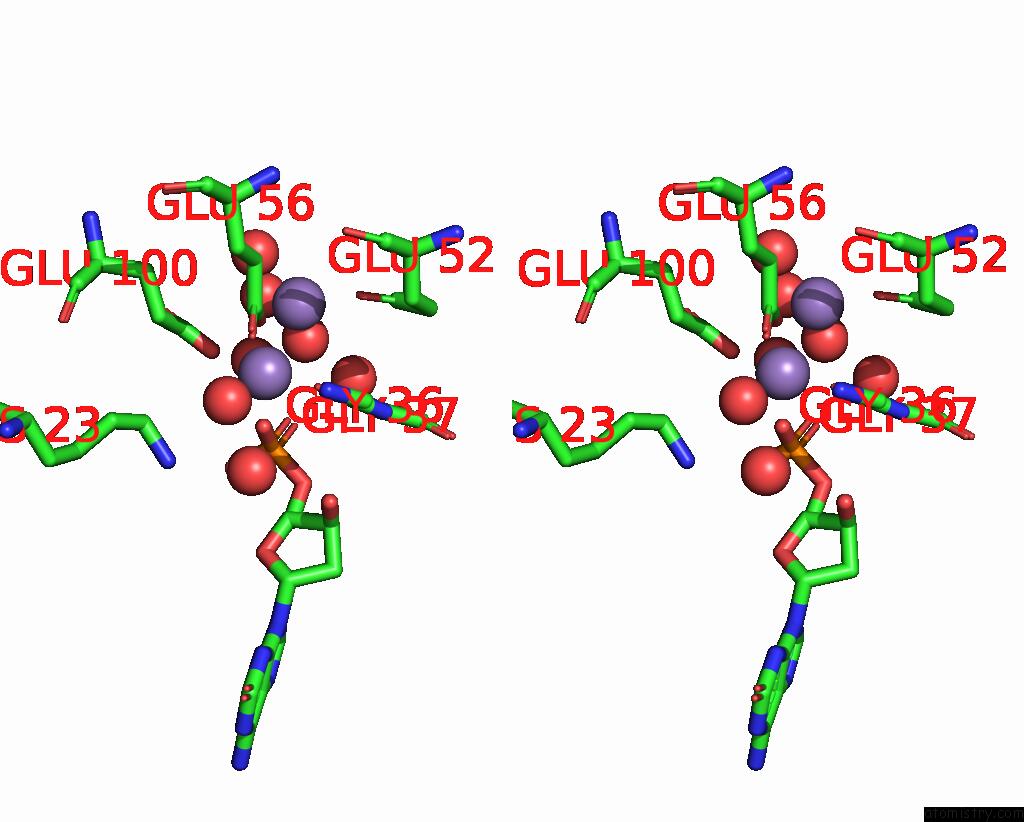
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+ within 5.0Å range:
|
Manganese binding site 2 out of 4 in 8i8t
Go back to
Manganese binding site 2 out
of 4 in the Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+
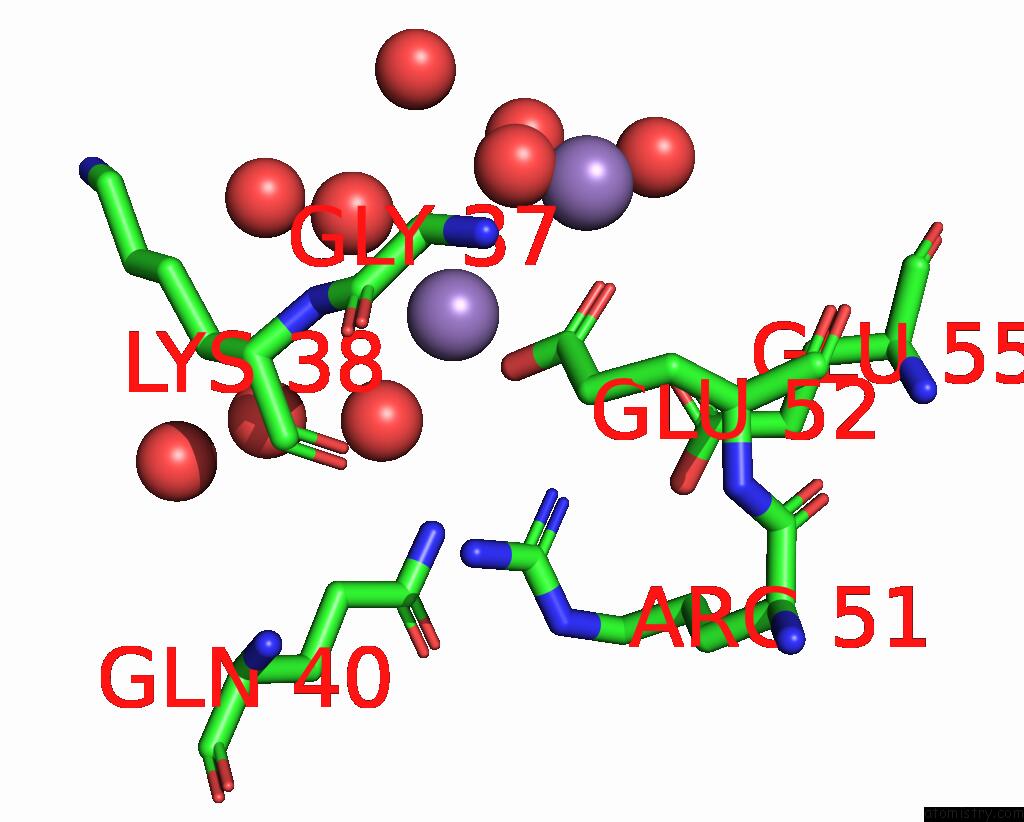
Mono view
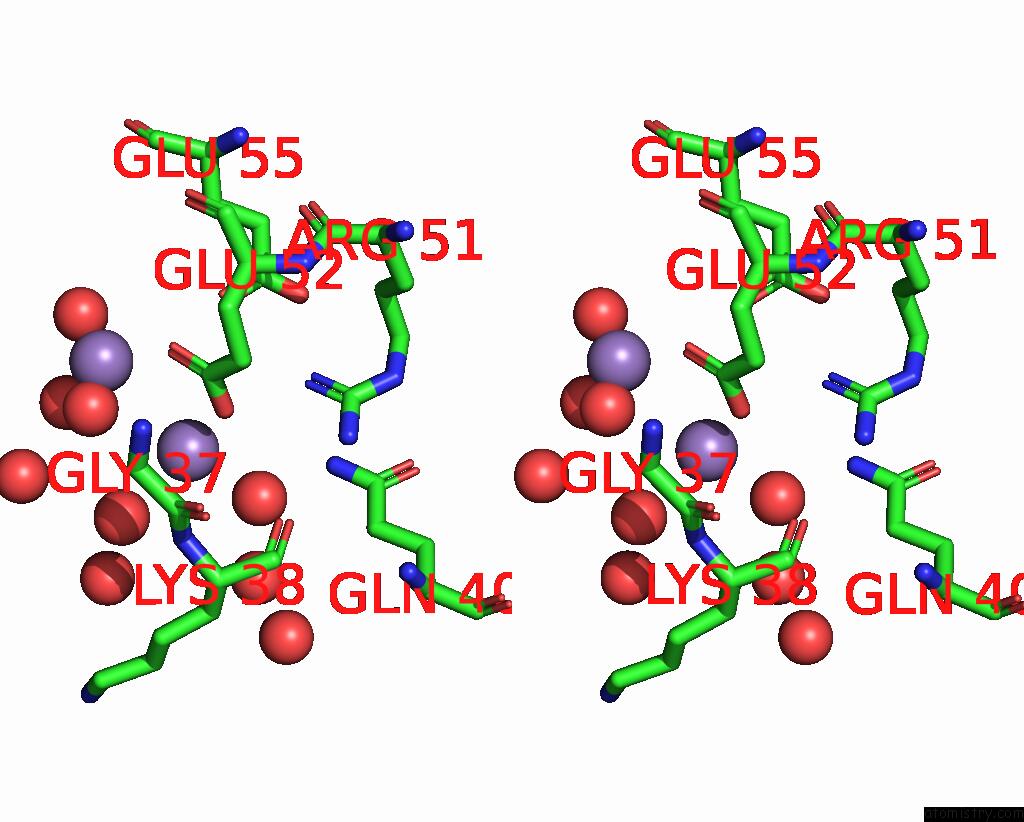
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+ within 5.0Å range:
|
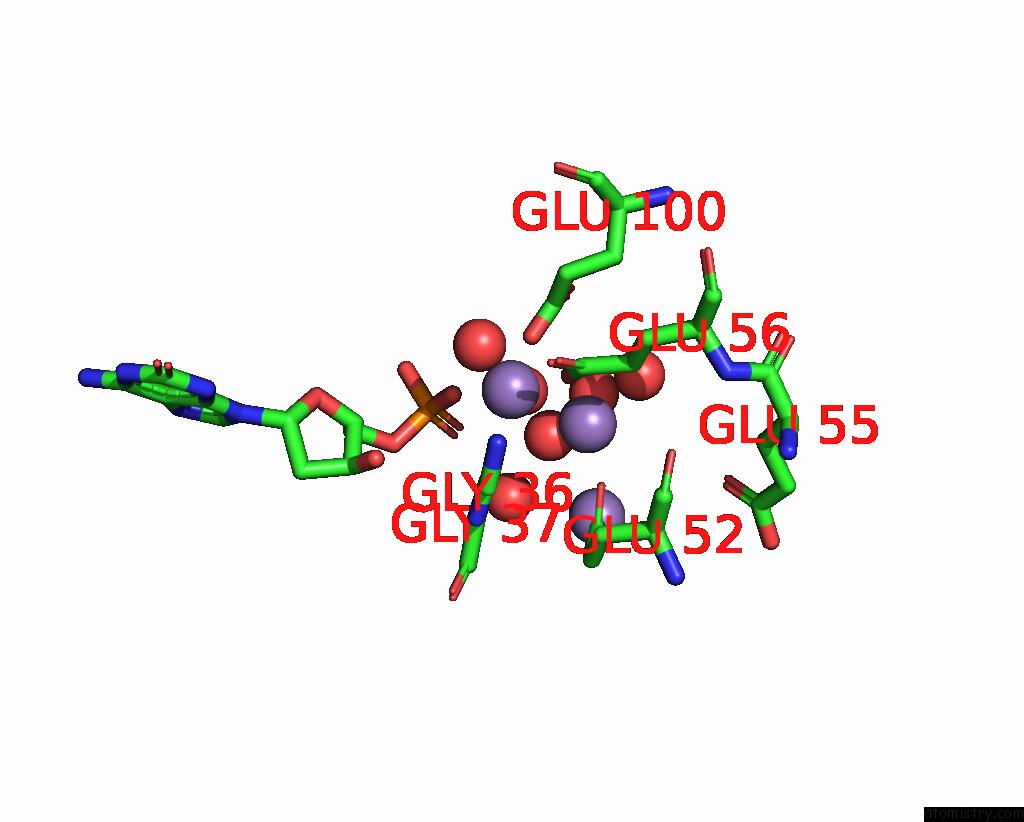
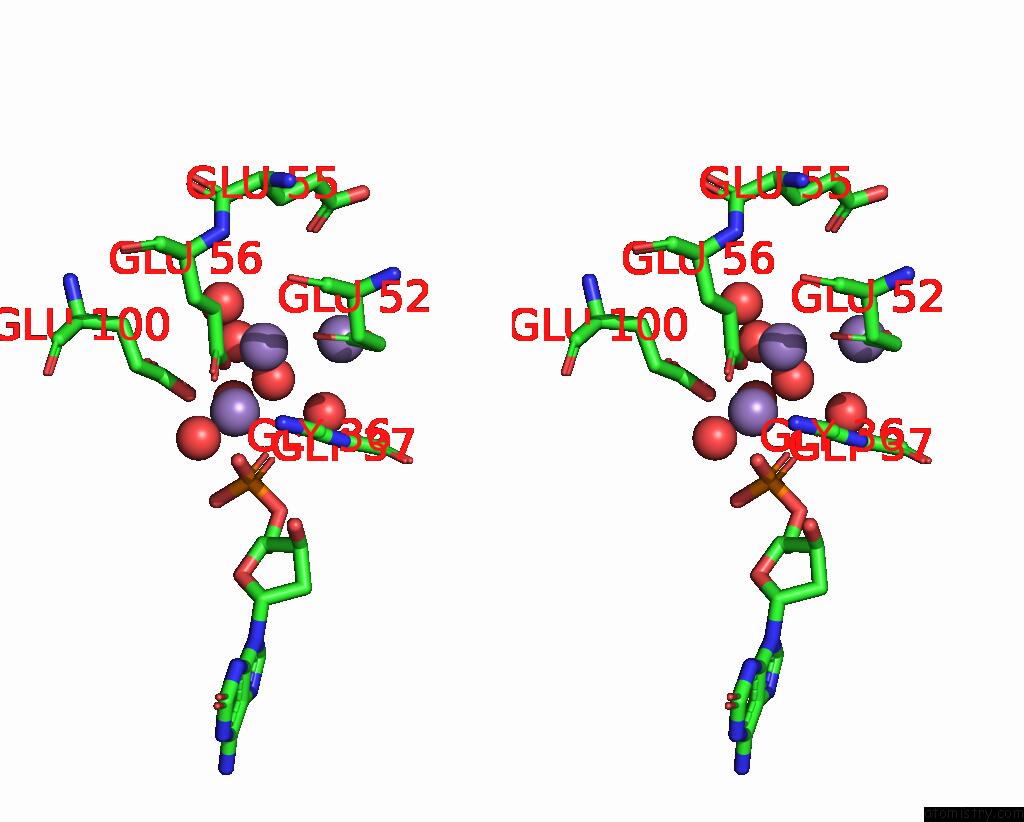
Manganese binding site 3 out of 4 in 8i8t
Go back to
Manganese binding site 3 out
of 4 in the Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+ within 5.0Å range:
|
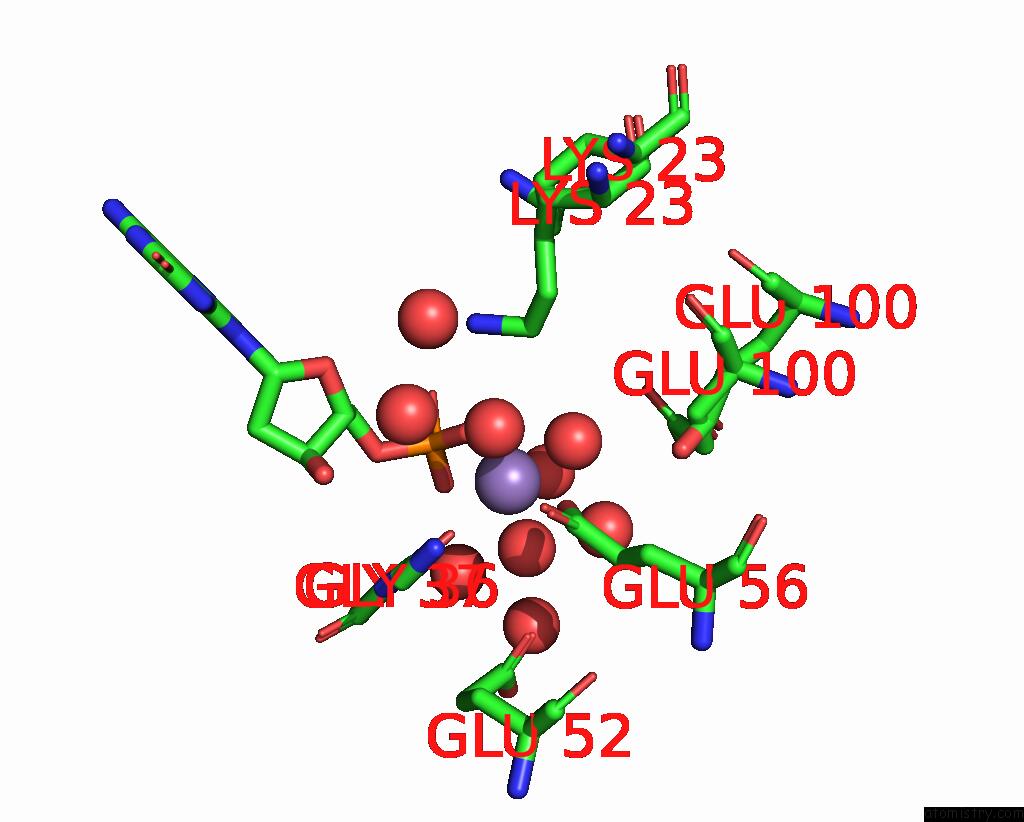
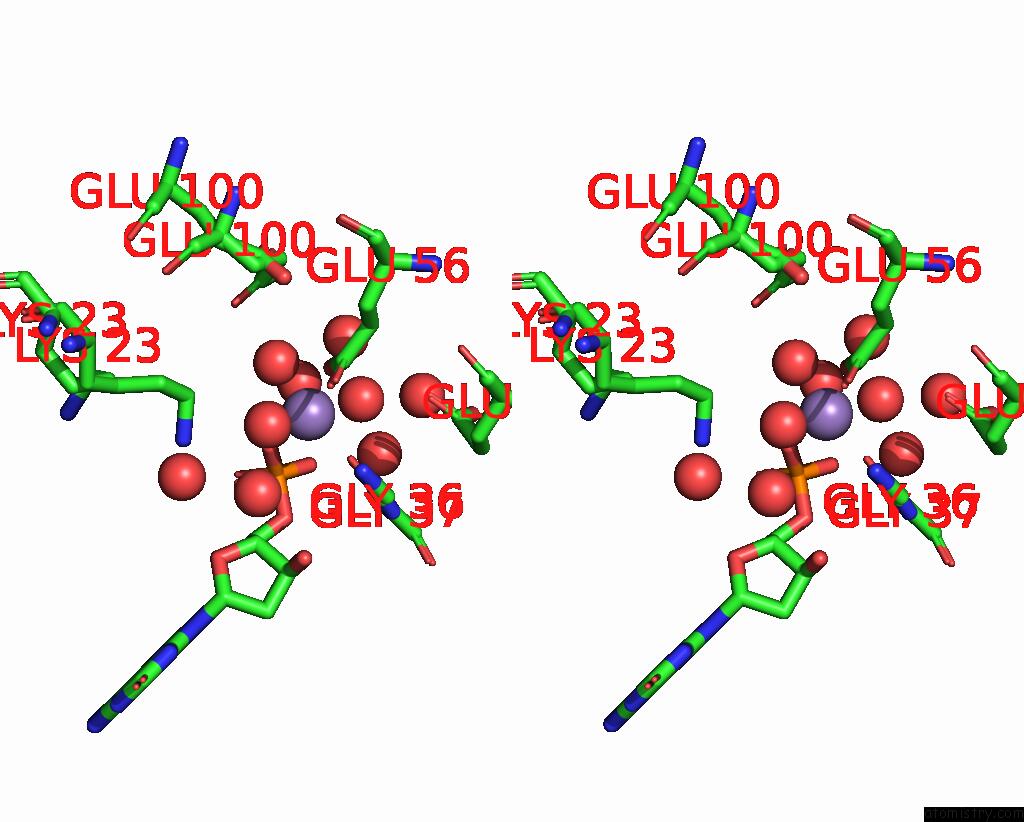
Manganese binding site 4 out of 4 in 8i8t
Go back to
Manganese binding site 4 out
of 4 in the Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of Crystal Structure of Human MTH1(G2K Mutant) in Complex with 2-Oxo-Damp and MN2+ within 5.0Å range:
|
Reference:
T.Nakamura,
Y.Koga-Ogawa,
K.Fujimiya,
M.Chirifu,
M.Goto,
S.Ikemizu,
Y.Nakabeppu,
Y.Yamagata.
Protonation States of Asp Residues in the Human Nudix Hydrolase MTH1 Contribute to Its Broad Substrate Recognition. Febs Lett. 2023.
ISSN: ISSN 0014-5793
PubMed: 36914375
DOI: 10.1002/1873-3468.14611
Page generated: Sun Oct 6 12:29:47 2024
ISSN: ISSN 0014-5793
PubMed: 36914375
DOI: 10.1002/1873-3468.14611
Last articles
Ca in 5T77Ca in 5T5P
Ca in 5T7P
Ca in 5SZR
Ca in 5T5N
Ca in 5T5L
Ca in 5T5J
Ca in 5T55
Ca in 5T54
Ca in 5T5I