Manganese »
PDB 7z03-8awv »
7zz6 »
Manganese in PDB 7zz6: Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa
Enzymatic activity of Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa
All present enzymatic activity of Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa:
6.4.1.1;
6.4.1.1;
Other elements in 7zz6:
The structure of Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa also contains other interesting chemical elements:
| Magnesium | (Mg) | 2 atoms |
Manganese Binding Sites:
The binding sites of Manganese atom in the Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa
(pdb code 7zz6). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa, PDB code: 7zz6:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa, PDB code: 7zz6:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 7zz6
Go back to
Manganese binding site 1 out
of 2 in the Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa

Mono view
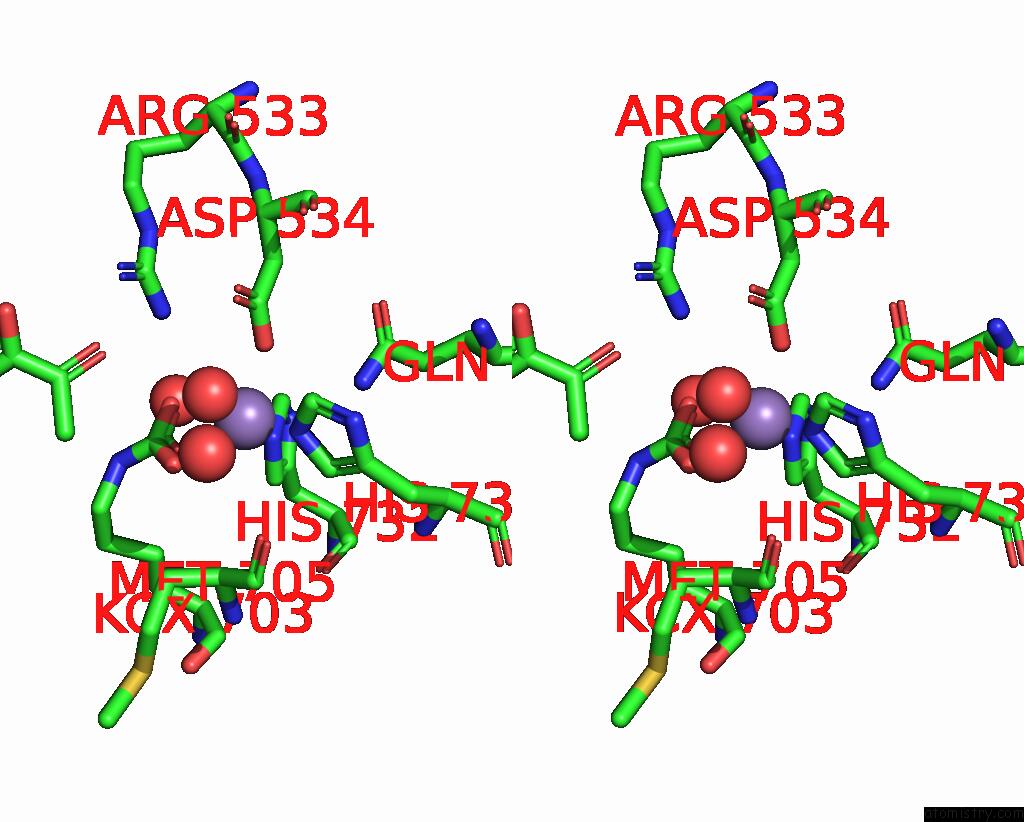
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa within 5.0Å range:
|
Manganese binding site 2 out of 2 in 7zz6
Go back to
Manganese binding site 2 out
of 2 in the Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa
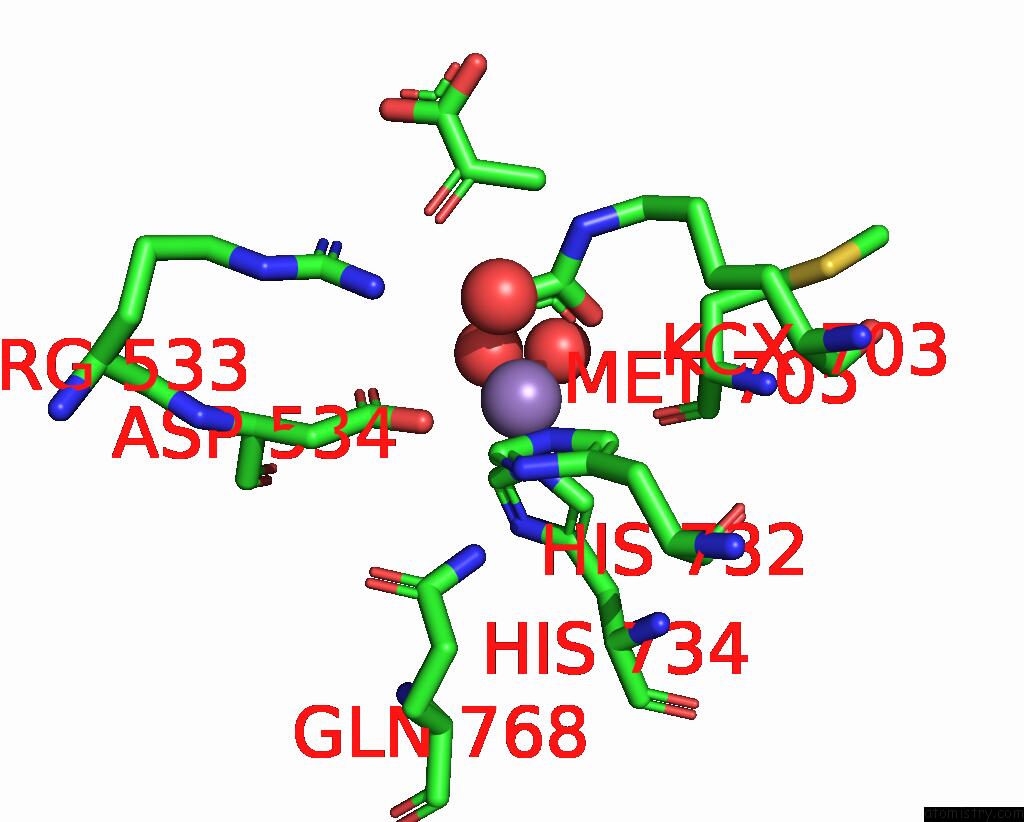
Mono view
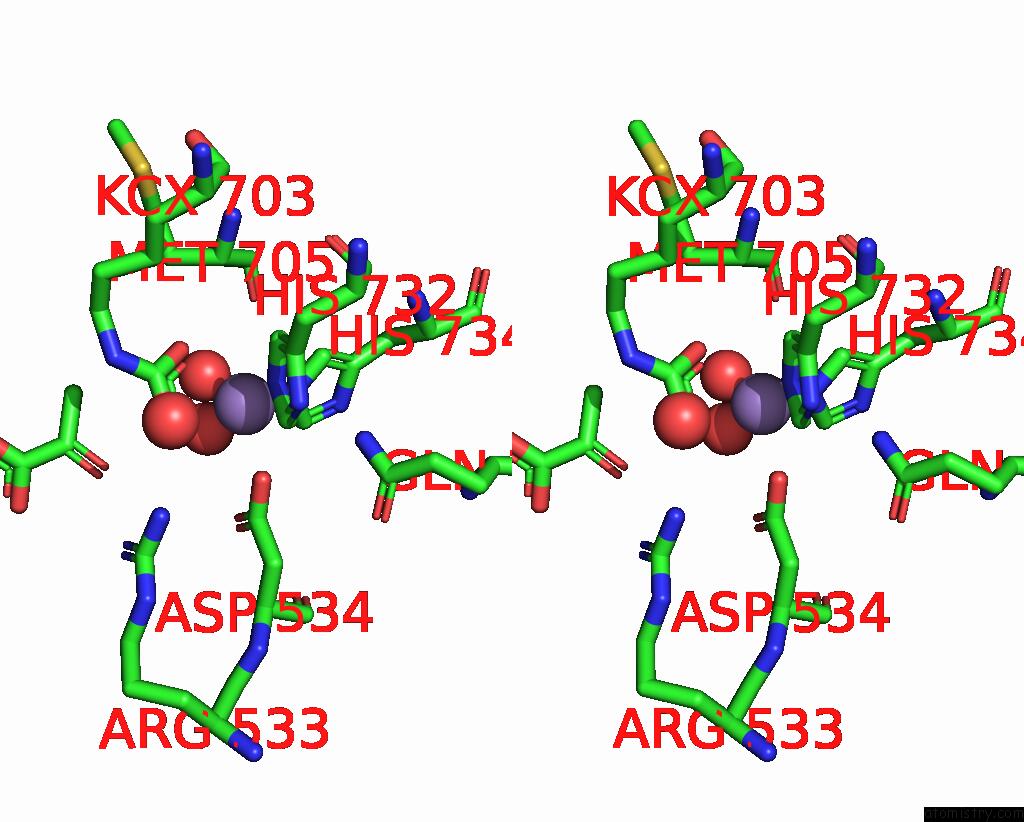
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Cryo-Em Structure of "Ct-Ct Dimer" of Lactococcus Lactis Pyruvate Carboxylase with Acetyl-Coa within 5.0Å range:
|
Reference:
J.P.Lopez-Alonso,
M.Lazaro,
D.Gil-Carton,
P.H.Choi,
A.Dodu,
L.Tong,
M.Valle.
Cryoem Structural Exploration of Catalytically Active Enzyme Pyruvate Carboxylase. Nat Commun V. 13 6185 2022.
ISSN: ESSN 2041-1723
PubMed: 36261450
DOI: 10.1038/S41467-022-33987-2
Page generated: Sun Oct 6 11:15:17 2024
ISSN: ESSN 2041-1723
PubMed: 36261450
DOI: 10.1038/S41467-022-33987-2
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO