Manganese »
PDB 7x9j-7yzp »
7xrf »
Manganese in PDB 7xrf: Crystal Structaure of Dgpb/C Complex
Protein crystallography data
The structure of Crystal Structaure of Dgpb/C Complex, PDB code: 7xrf
was solved by
M.Ma,
P.He,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.84 / 2.14 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 78.076, 159.704, 176.168, 90, 90, 90 |
| R / Rfree (%) | 20.6 / 25.1 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Crystal Structaure of Dgpb/C Complex
(pdb code 7xrf). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 4 binding sites of Manganese where determined in the Crystal Structaure of Dgpb/C Complex, PDB code: 7xrf:
Jump to Manganese binding site number: 1; 2; 3; 4;
In total 4 binding sites of Manganese where determined in the Crystal Structaure of Dgpb/C Complex, PDB code: 7xrf:
Jump to Manganese binding site number: 1; 2; 3; 4;
Manganese binding site 1 out of 4 in 7xrf
Go back to
Manganese binding site 1 out
of 4 in the Crystal Structaure of Dgpb/C Complex
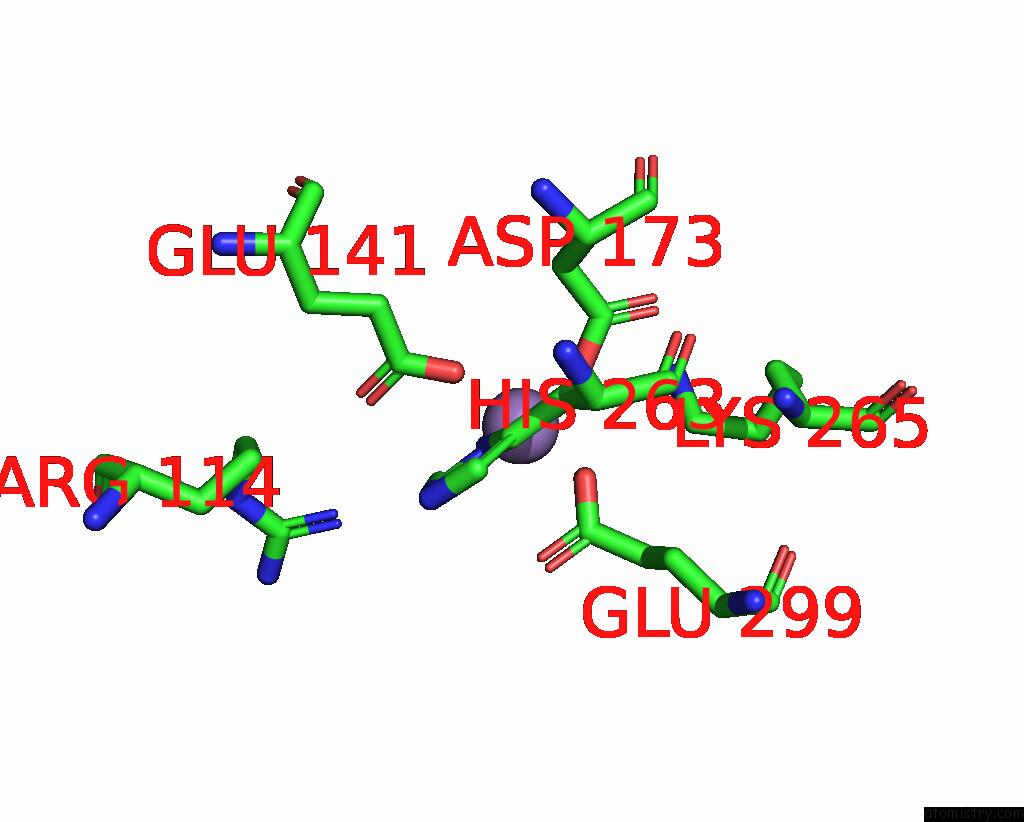
Mono view
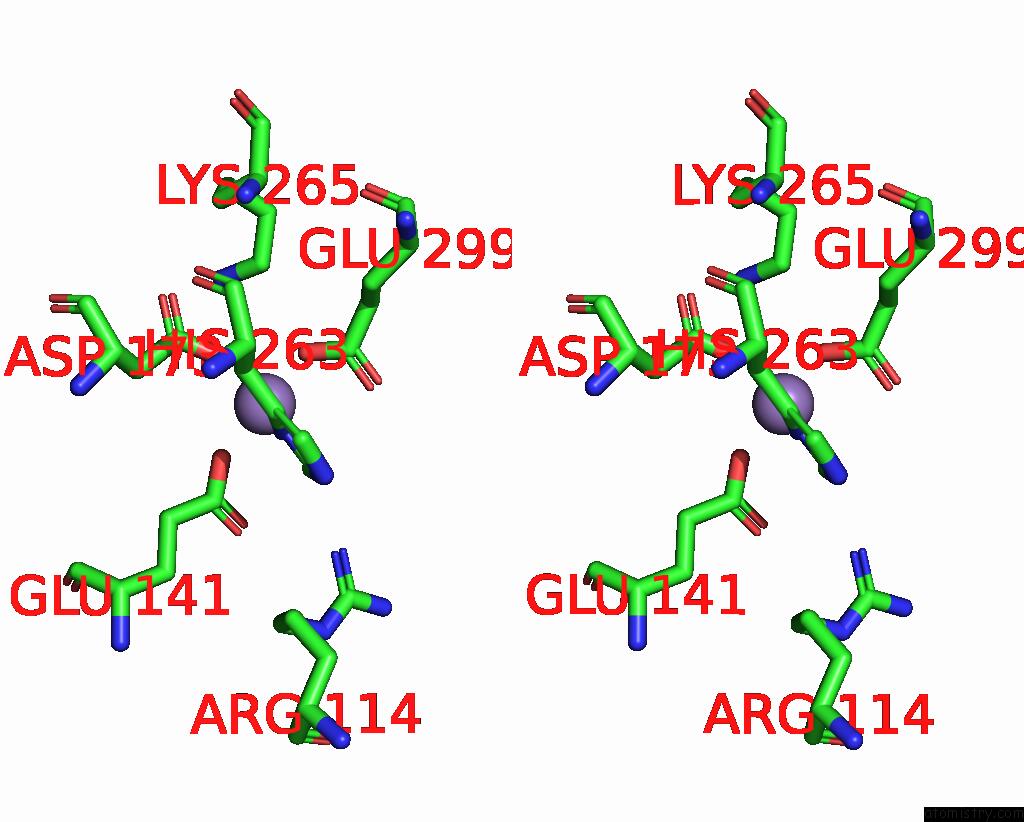
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Crystal Structaure of Dgpb/C Complex within 5.0Å range:
|
Manganese binding site 2 out of 4 in 7xrf
Go back to
Manganese binding site 2 out
of 4 in the Crystal Structaure of Dgpb/C Complex

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Crystal Structaure of Dgpb/C Complex within 5.0Å range:
|
Manganese binding site 3 out of 4 in 7xrf
Go back to
Manganese binding site 3 out
of 4 in the Crystal Structaure of Dgpb/C Complex
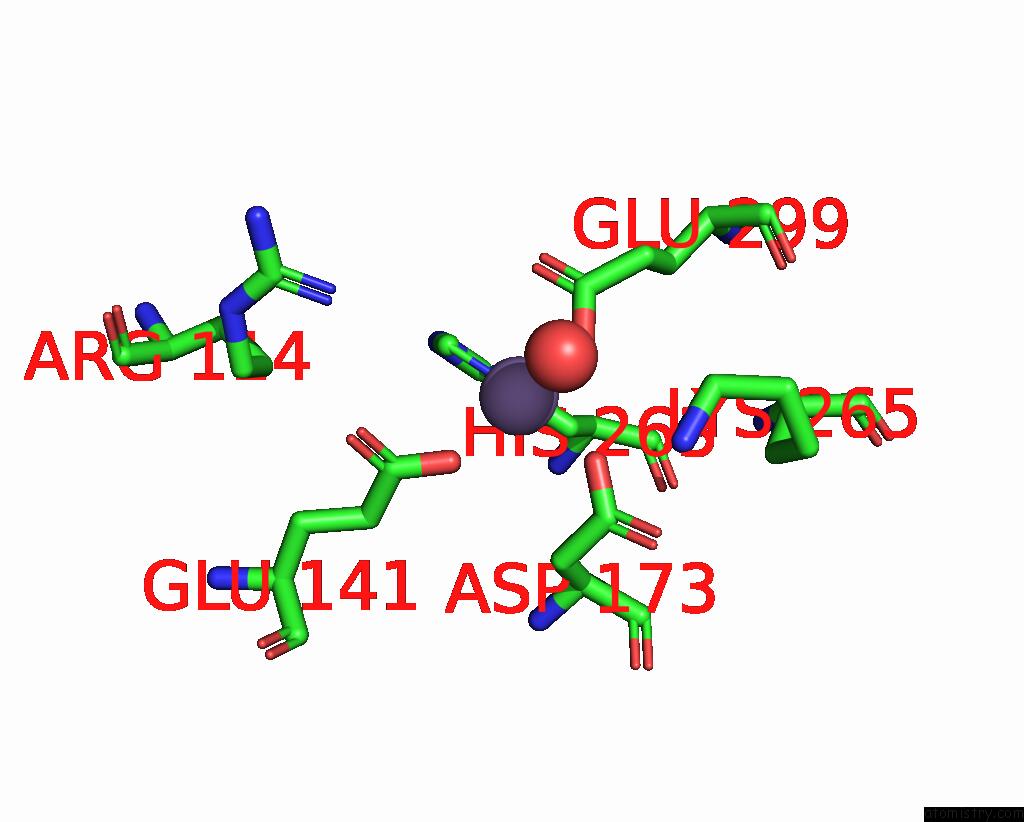
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Crystal Structaure of Dgpb/C Complex within 5.0Å range:
|
Manganese binding site 4 out of 4 in 7xrf
Go back to
Manganese binding site 4 out
of 4 in the Crystal Structaure of Dgpb/C Complex
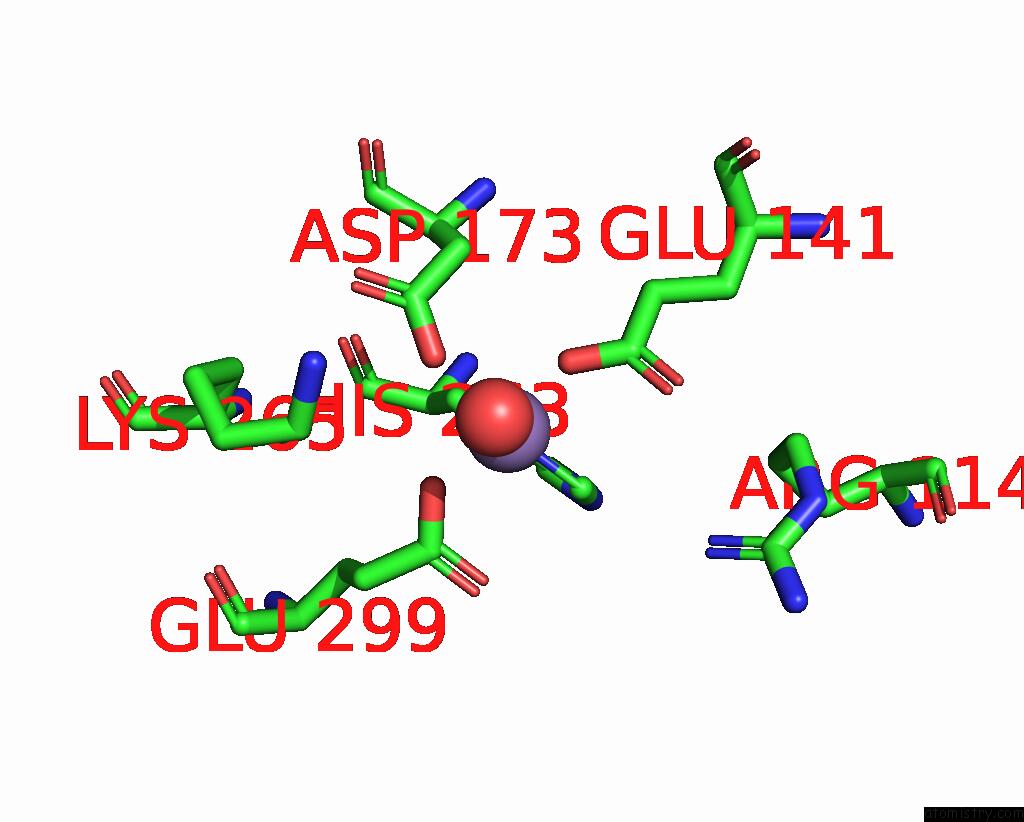
Mono view
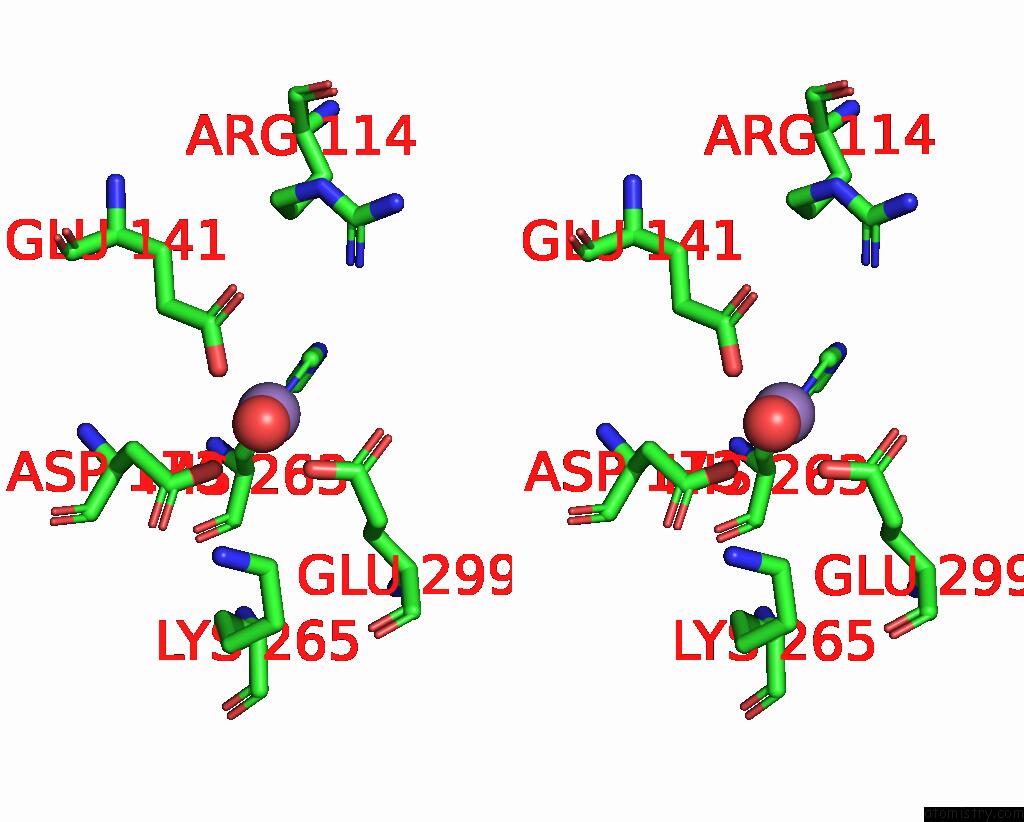
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of Crystal Structaure of Dgpb/C Complex within 5.0Å range:
|
Reference:
P.He,
S.Wang,
S.Li,
S.Liu,
S.Zhou,
J.Wang,
J.Tao,
D.Wang,
R.Wang,
W.Ma.
Structural Mechanism of A Dual-Functional Enzyme Dgpa/B/C As Both A C-Glycoside Cleaving Enzyme and An O- to C-Glycoside Isomerase. Acta Pharm Sin B V. 13 246 2023.
ISSN: ESSN 2211-3843
DOI: 10.1016/J.APSB.2022.05.022
Page generated: Sun Oct 6 11:07:43 2024
ISSN: ESSN 2211-3843
DOI: 10.1016/J.APSB.2022.05.022
Last articles
F in 5AVLF in 5APK
F in 5AR8
F in 5ARG
F in 5AOR
F in 5ARF
F in 5APJ
F in 5AR4
F in 5AP7
F in 5APH