Manganese »
PDB 6rwz-6txf »
6s2t »
Manganese in PDB 6s2t: Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp
Enzymatic activity of Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp
All present enzymatic activity of Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp:
2.7.6.5;
2.7.6.5;
Protein crystallography data
The structure of Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp, PDB code: 6s2t
was solved by
A.Garcia-Pino,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 62.17 / 2.75 |
| Space group | P 41 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 87.927, 87.927, 184.252, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.6 / 22 |
Other elements in 6s2t:
The structure of Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
| Chlorine | (Cl) | 1 atom |
| Sodium | (Na) | 1 atom |
Manganese Binding Sites:
The binding sites of Manganese atom in the Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp
(pdb code 6s2t). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total only one binding site of Manganese was determined in the Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp, PDB code: 6s2t:
In total only one binding site of Manganese was determined in the Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp, PDB code: 6s2t:
Manganese binding site 1 out of 1 in 6s2t
Go back to
Manganese binding site 1 out
of 1 in the Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp
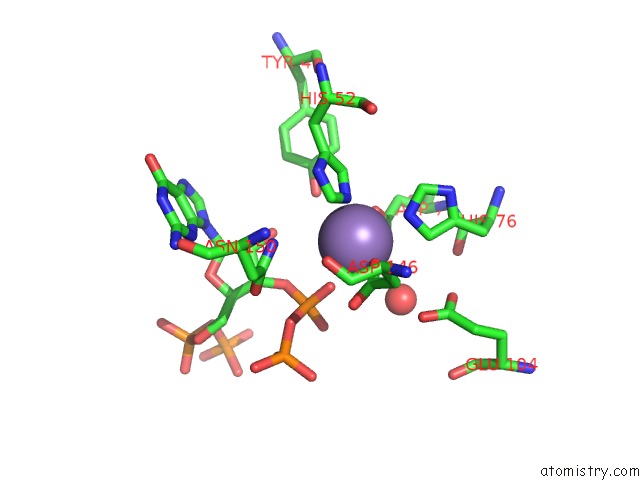
Mono view
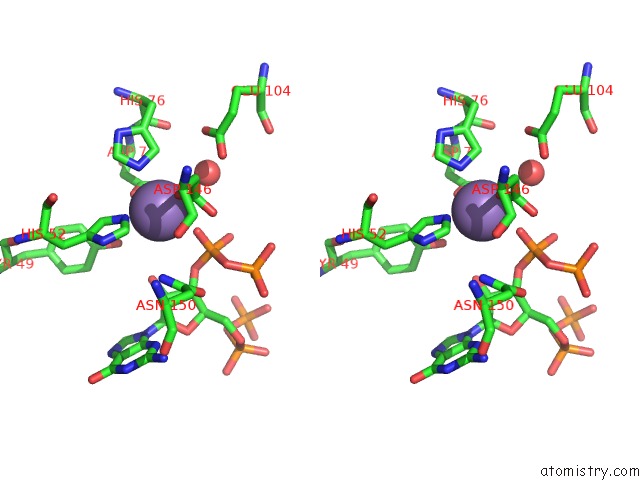
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Structure of the N-Terminal Catalytic Region of T. Thermophilus Rel Bound to Ppgpp within 5.0Å range:
|
Reference:
H.Tamman,
K.Van Nerom,
H.Takada,
N.Vandenberk,
D.Scholl,
Y.Polikanov,
J.Hofkens,
A.Talavera,
V.Hauryliuk,
J.Hendrix,
A.Garcia-Pino.
A Nucleotide-Switch Mechanism Mediates Opposing Catalytic Activities of Rel Enzymes. Nat.Chem.Biol. V. 16 834 2020.
ISSN: ESSN 1552-4469
PubMed: 32393900
DOI: 10.1038/S41589-020-0520-2
Page generated: Sun Oct 6 07:05:10 2024
ISSN: ESSN 1552-4469
PubMed: 32393900
DOI: 10.1038/S41589-020-0520-2
Last articles
Cl in 5WN1Cl in 5WMM
Cl in 5WLO
Cl in 5WL6
Cl in 5WMD
Cl in 5WLY
Cl in 5WL3
Cl in 5WL7
Cl in 5WLG
Cl in 5WL5