Manganese »
PDB 6qv9-6ru4 »
6r4s »
Manganese in PDB 6r4s: Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp
Protein crystallography data
The structure of Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp, PDB code: 6r4s
was solved by
M.L.Kilkenny,
L.Pellegrini,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.32 / 2.75 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 110.130, 117.270, 151.230, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22.8 / 26.9 |
Other elements in 6r4s:
The structure of Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp also contains other interesting chemical elements:
| Zinc | (Zn) | 2 atoms |
Manganese Binding Sites:
The binding sites of Manganese atom in the Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp
(pdb code 6r4s). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 4 binding sites of Manganese where determined in the Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp, PDB code: 6r4s:
Jump to Manganese binding site number: 1; 2; 3; 4;
In total 4 binding sites of Manganese where determined in the Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp, PDB code: 6r4s:
Jump to Manganese binding site number: 1; 2; 3; 4;
Manganese binding site 1 out of 4 in 6r4s
Go back to
Manganese binding site 1 out
of 4 in the Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp
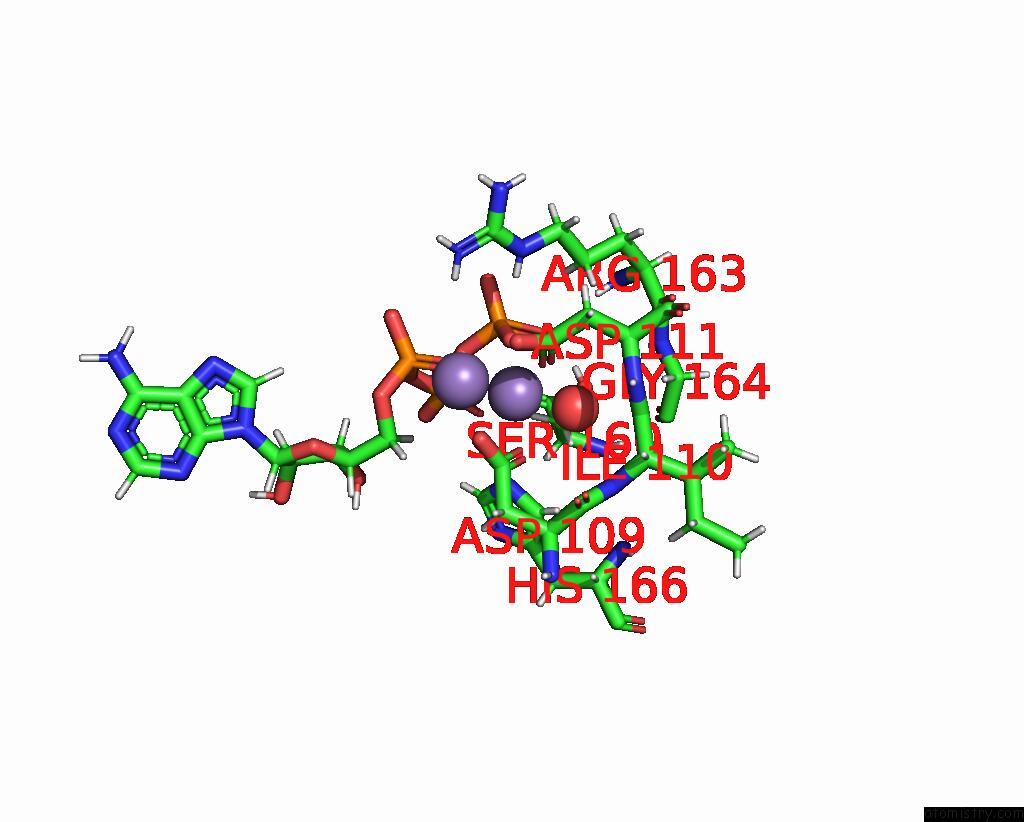
Mono view
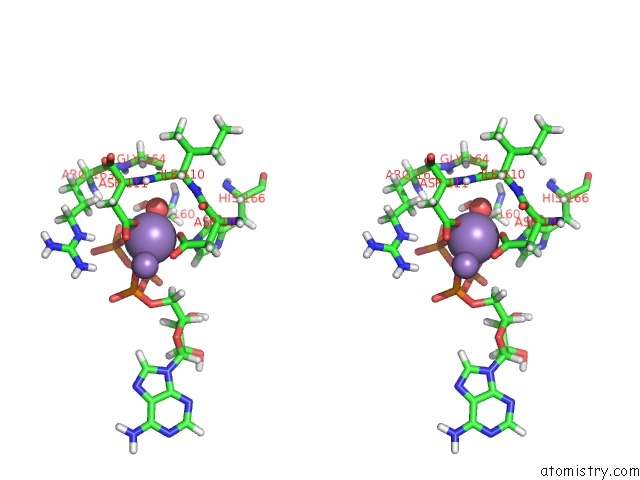
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp within 5.0Å range:
|
Manganese binding site 2 out of 4 in 6r4s
Go back to
Manganese binding site 2 out
of 4 in the Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp
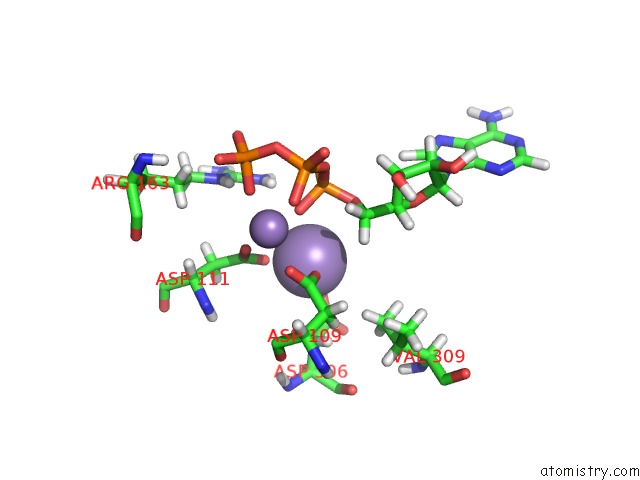
Mono view
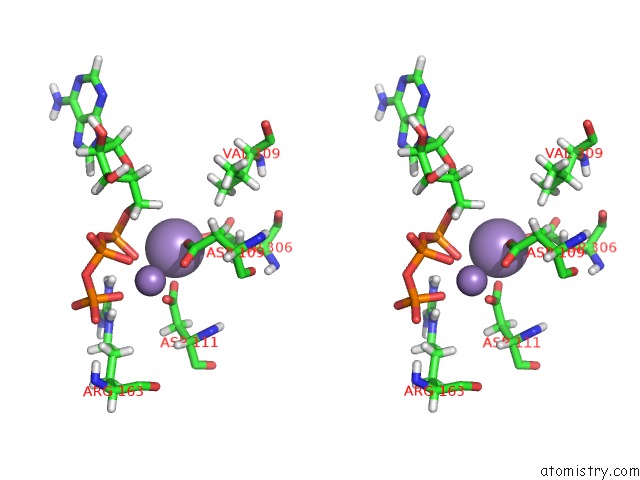
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp within 5.0Å range:
|
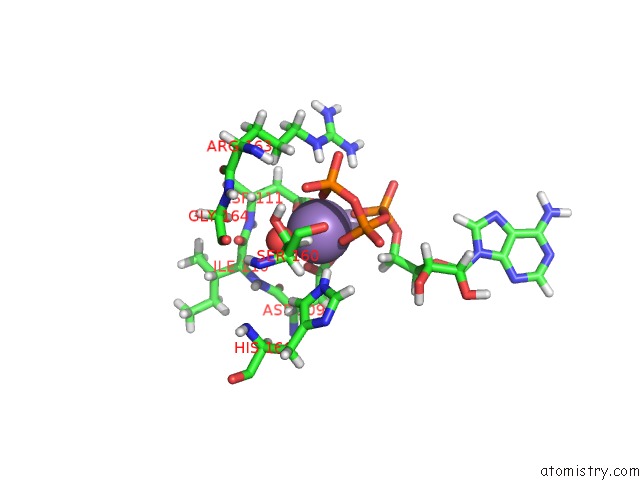
Manganese binding site 3 out of 4 in 6r4s
Go back to
Manganese binding site 3 out
of 4 in the Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp
Mono view
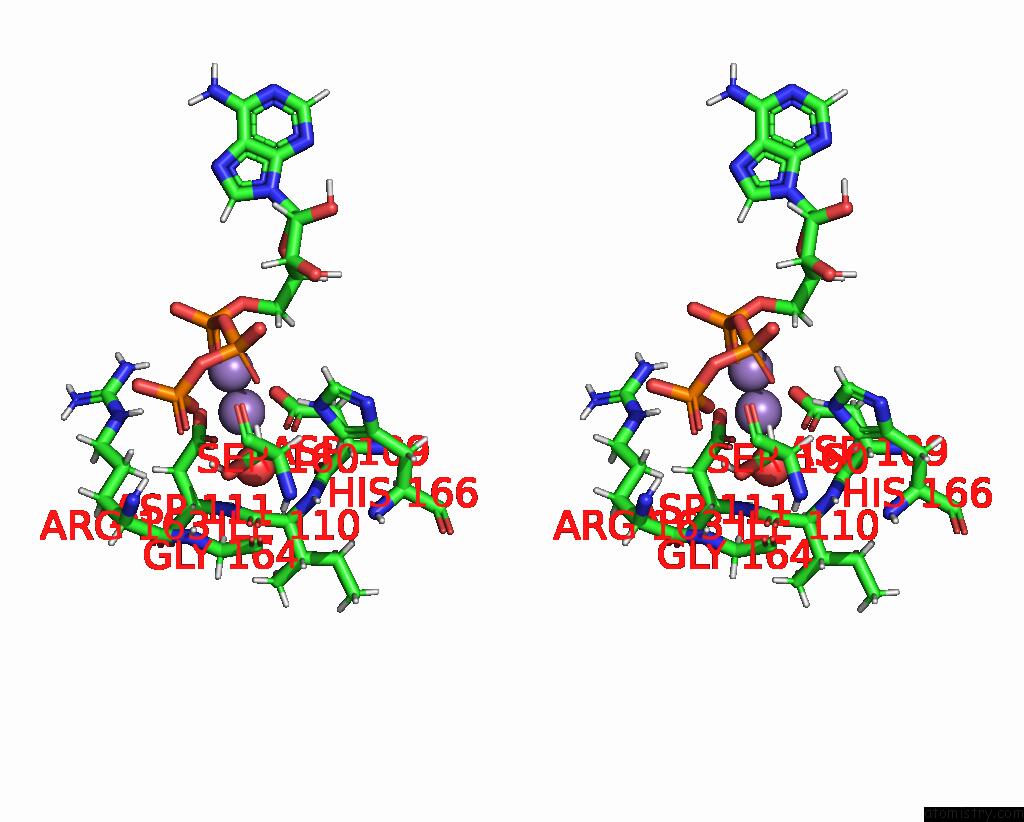
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp within 5.0Å range:
|
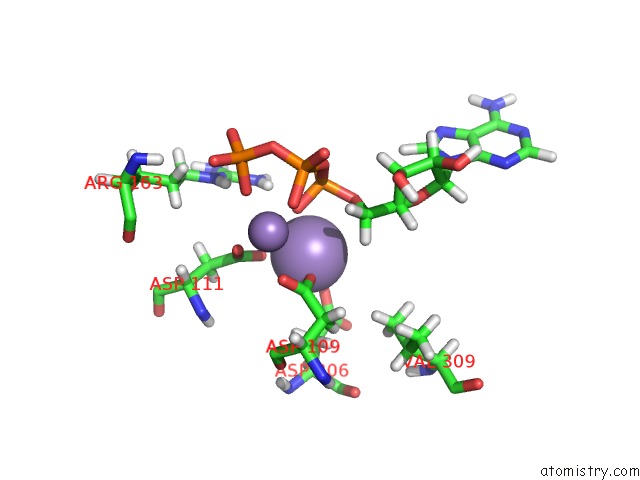
Manganese binding site 4 out of 4 in 6r4s
Go back to
Manganese binding site 4 out
of 4 in the Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp
Mono view
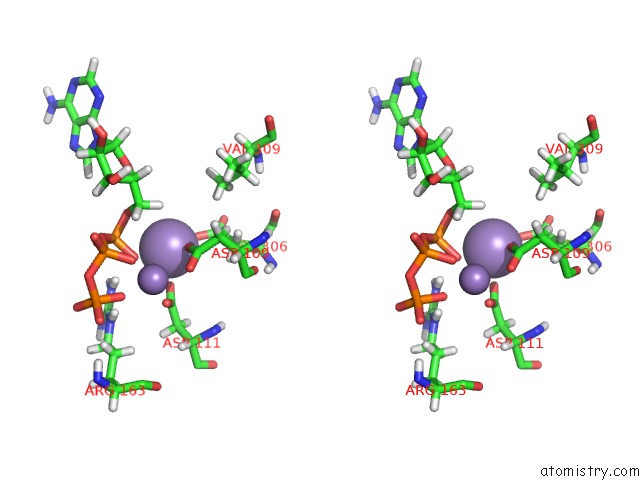
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of Crystal Structure of the PRI1 Subunit of Human Primase Bound to Atp within 5.0Å range:
|
Reference:
S.Holzer,
N.J.Rzechorzek,
I.R.Short,
M.Jenkyn-Bedford,
L.Pellegrini,
M.L.Kilkenny.
Structural Basis For Inhibition of Human Primase By Arabinofuranosyl Nucleoside Analogues Fludarabine and Vidarabine. Acs Chem.Biol. V. 14 1904 2019.
ISSN: ESSN 1554-8937
PubMed: 31479243
DOI: 10.1021/ACSCHEMBIO.9B00367
Page generated: Sun Oct 6 06:59:40 2024
ISSN: ESSN 1554-8937
PubMed: 31479243
DOI: 10.1021/ACSCHEMBIO.9B00367
Last articles
Ca in 5VT8Ca in 5VYF
Ca in 5VYB
Ca in 5VXZ
Ca in 5VTM
Ca in 5VWM
Ca in 5VTD
Ca in 5VUG
Ca in 5VS1
Ca in 5VRB