Manganese »
PDB 5uqt-5vpx »
5vcm »
Manganese in PDB 5vcm: Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese
Enzymatic activity of Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese
All present enzymatic activity of Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese:
2.4.1.143;
2.4.1.143;
Protein crystallography data
The structure of Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese, PDB code: 5vcm
was solved by
J.H.Sanders,
R.Kadirvelraj,
Z.A.Wood,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 43.04 / 1.60 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 46.130, 139.330, 63.210, 90.00, 111.08, 90.00 |
| R / Rfree (%) | 18.6 / 22 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese
(pdb code 5vcm). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese, PDB code: 5vcm:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese, PDB code: 5vcm:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 5vcm
Go back to
Manganese binding site 1 out
of 2 in the Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese
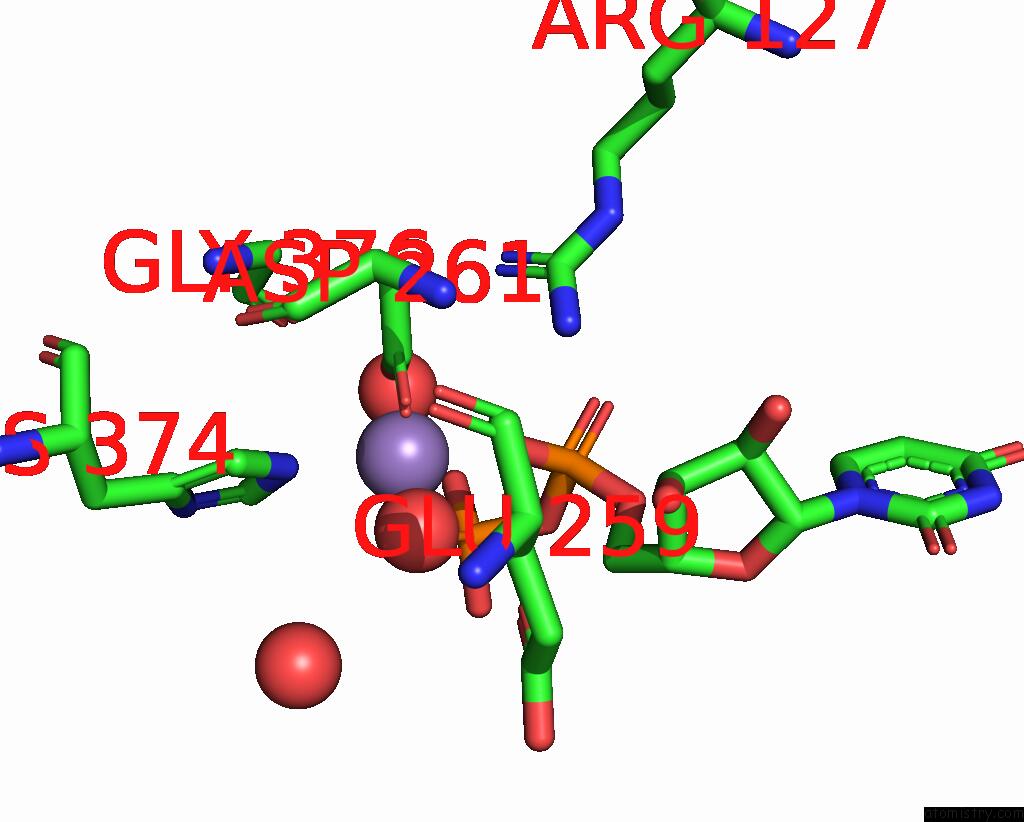
Mono view
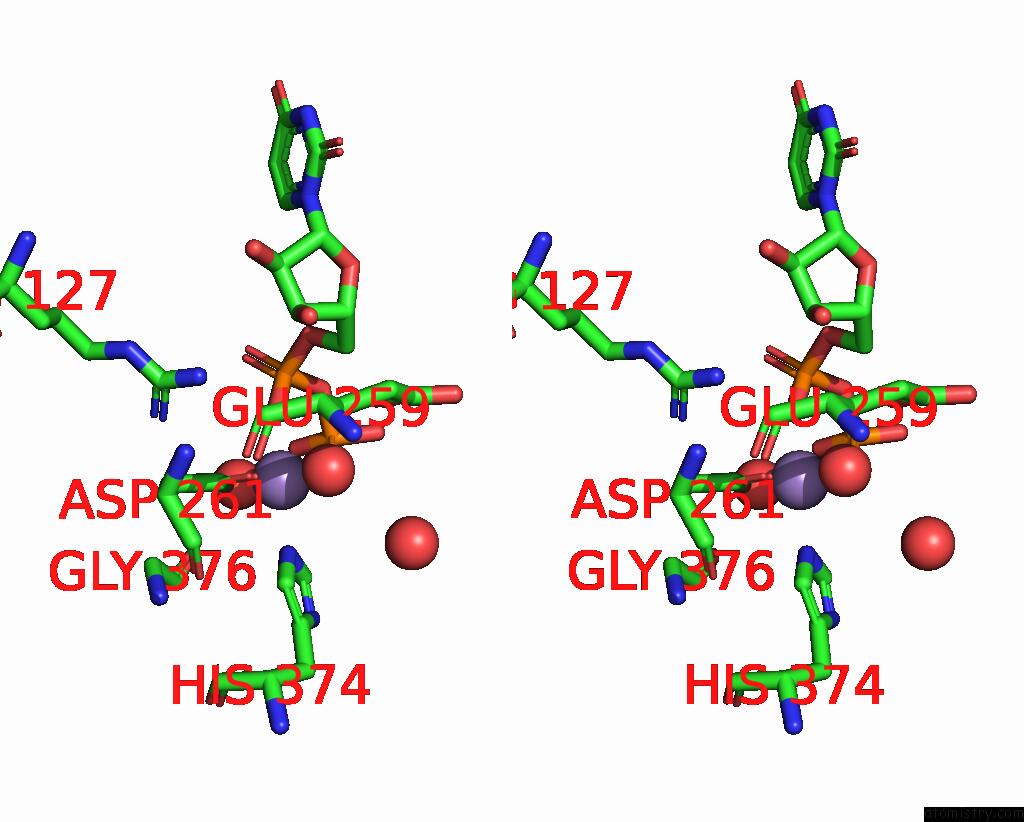
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese within 5.0Å range:
|
Manganese binding site 2 out of 2 in 5vcm
Go back to
Manganese binding site 2 out
of 2 in the Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese

Mono view
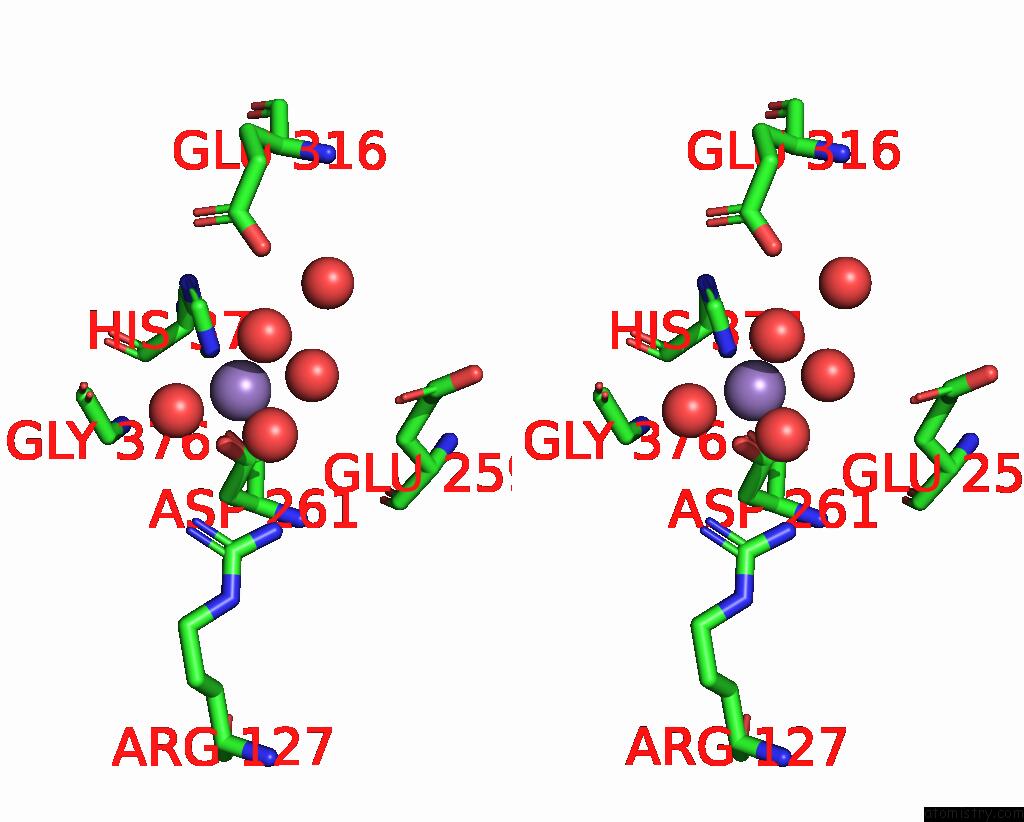
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Alpha-1,6-Mannosyl-Glycoprotein 2-Beta-N-Acetylglucosaminyltransferase with Bound Udp and Manganese within 5.0Å range:
|
Reference:
R.Kadirvelraj,
J.Y.Yang,
J.H.Sanders,
L.Liu,
A.Ramiah,
P.K.Prabhakar,
G.J.Boons,
Z.A.Wood,
K.W.Moremen.
Humann-Acetylglucosaminyltransferase II Substrate Recognition Uses A Modular Architecture That Includes A Convergent Exosite. Proc. Natl. Acad. Sci. V. 115 4637 2018U.S.A..
ISSN: ESSN 1091-6490
PubMed: 29666272
DOI: 10.1073/PNAS.1716988115
Page generated: Sat Aug 16 19:23:22 2025
ISSN: ESSN 1091-6490
PubMed: 29666272
DOI: 10.1073/PNAS.1716988115
Last articles
Mo in 8BQTMo in 8CCQ
Mo in 8BTS
Mo in 8BQR
Mo in 8BQQ
Mo in 8BQP
Mo in 7Z5J
Mo in 7ZUB
Mo in 7Z0T
Mo in 7WY3