Manganese »
PDB 5cdn-5dcb »
5ckv »
Manganese in PDB 5ckv: Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan
Enzymatic activity of Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan
All present enzymatic activity of Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan:
2.5.1.54;
2.5.1.54;
Protein crystallography data
The structure of Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan, PDB code: 5ckv
was solved by
S.Munack,
U.Krengel,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.30 / 2.79 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 204.760, 204.760, 66.774, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 17.5 / 22.6 |
Other elements in 5ckv:
The structure of Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Manganese Binding Sites:
The binding sites of Manganese atom in the Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan
(pdb code 5ckv). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan, PDB code: 5ckv:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan, PDB code: 5ckv:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 5ckv
Go back to
Manganese binding site 1 out
of 2 in the Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan
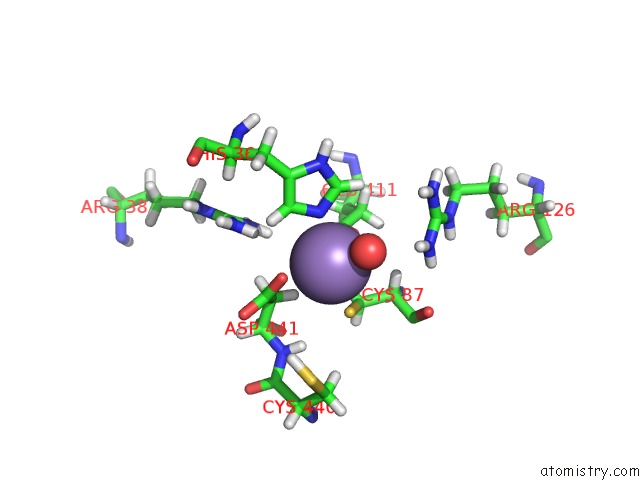
Mono view
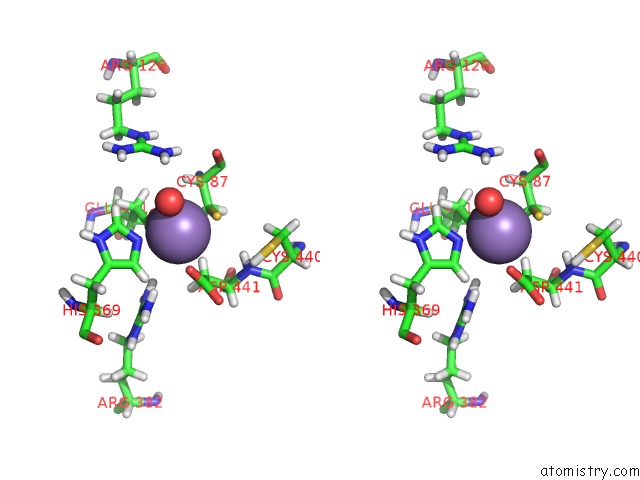
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan within 5.0Å range:
|
Manganese binding site 2 out of 2 in 5ckv
Go back to
Manganese binding site 2 out
of 2 in the Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan
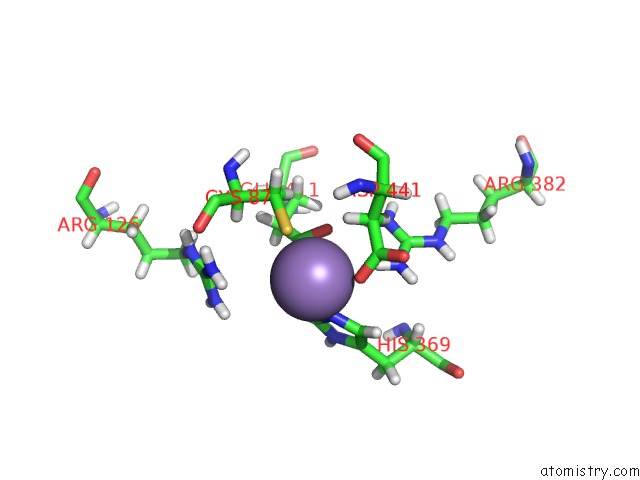
Mono view
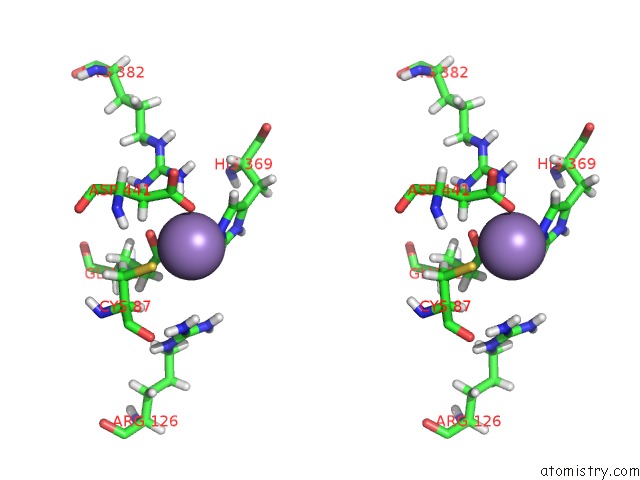
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Dahp Synthase From Mycobacterium Tuberculosis, Fully Inhibited By Tyrosine, Phenylalanine, and Tryptophan within 5.0Å range:
|
Reference:
S.Munack,
K.Roderer,
M.Okvist,
J.Kamarauskaite,
S.Sasso,
A.Van Eerde,
P.Kast,
U.Krengel.
Remote Control By Inter-Enzyme Allostery: A Novel Paradigm For Regulation of the Shikimate Pathway. J.Mol.Biol. V. 428 1237 2016.
ISSN: ESSN 1089-8638
PubMed: 26776476
DOI: 10.1016/J.JMB.2016.01.001
Page generated: Sat Oct 5 23:47:19 2024
ISSN: ESSN 1089-8638
PubMed: 26776476
DOI: 10.1016/J.JMB.2016.01.001
Last articles
Ca in 5TISCa in 5TI8
Ca in 5THG
Ca in 5TGX
Ca in 5TFK
Ca in 5TG3
Ca in 5TGQ
Ca in 5TGF
Ca in 5TFM
Ca in 5TEA