Manganese »
PDB 4u87-4wiu »
4ut3 »
Manganese in PDB 4ut3: X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide
Enzymatic activity of X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide
All present enzymatic activity of X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide:
3.1.3.16;
3.1.3.16;
Protein crystallography data
The structure of X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide, PDB code: 4ut3
was solved by
M.Zeh Silva,
J.Kopec,
D.Fotinou,
R.A.Steiner,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.40 / 2.19 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 38.450, 105.220, 89.820, 90.00, 90.34, 90.00 |
| R / Rfree (%) | 17.336 / 21.146 |
Manganese Binding Sites:
The binding sites of Manganese atom in the X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide
(pdb code 4ut3). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 4 binding sites of Manganese where determined in the X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide, PDB code: 4ut3:
Jump to Manganese binding site number: 1; 2; 3; 4;
In total 4 binding sites of Manganese where determined in the X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide, PDB code: 4ut3:
Jump to Manganese binding site number: 1; 2; 3; 4;
Manganese binding site 1 out of 4 in 4ut3
Go back to
Manganese binding site 1 out
of 4 in the X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide
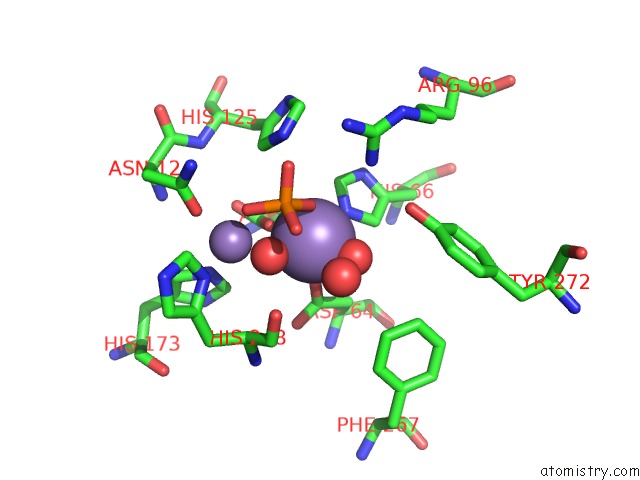
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide within 5.0Å range:
|
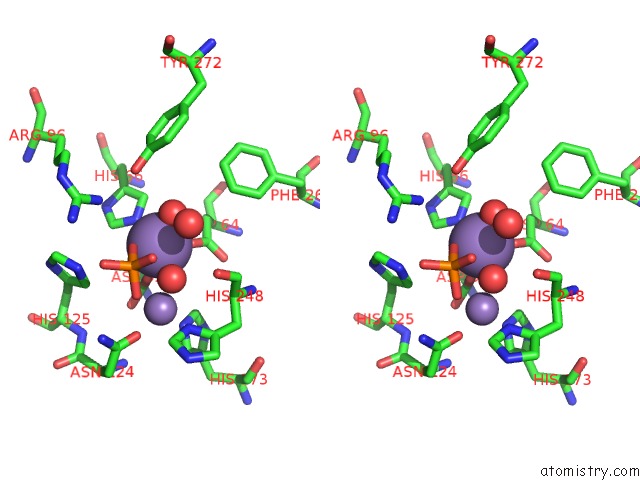
Manganese binding site 2 out of 4 in 4ut3
Go back to
Manganese binding site 2 out
of 4 in the X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide within 5.0Å range:
|
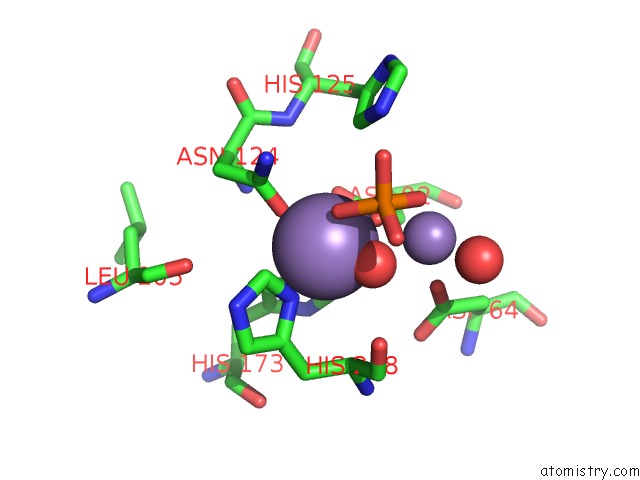
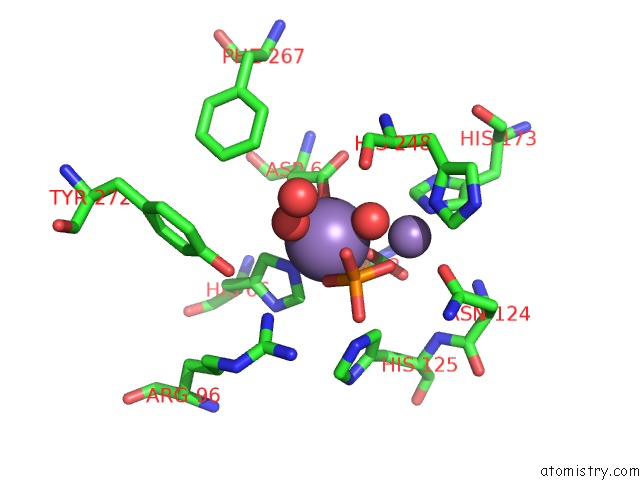
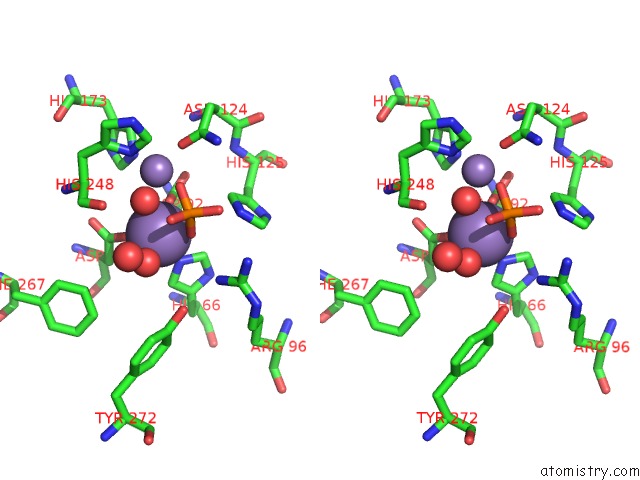
Manganese binding site 3 out of 4 in 4ut3
Go back to
Manganese binding site 3 out
of 4 in the X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide within 5.0Å range:
|
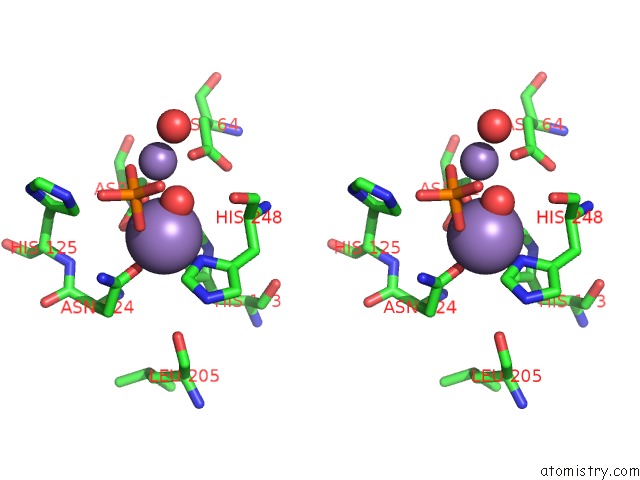
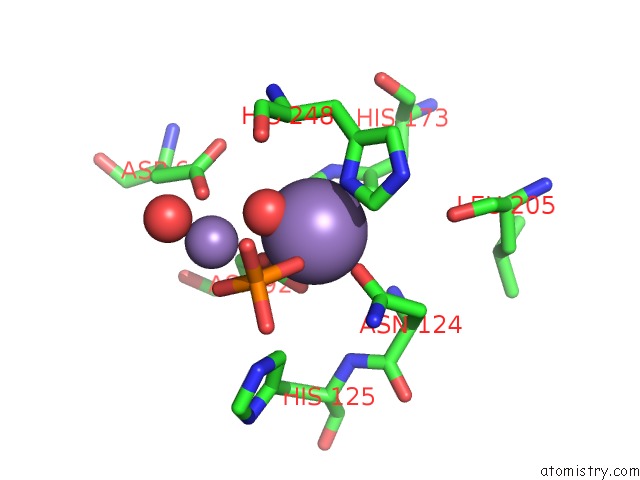
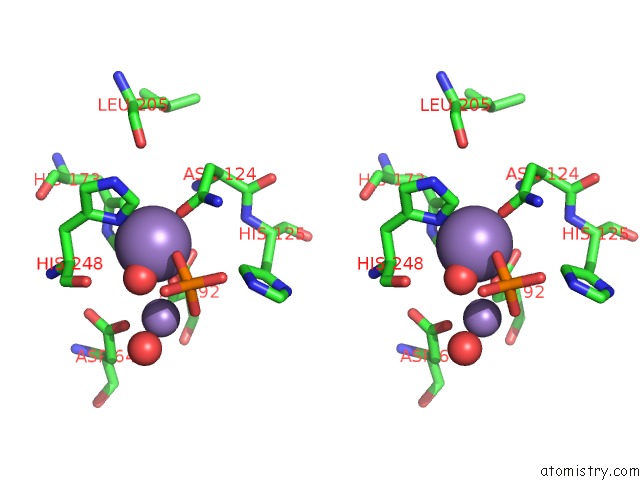
Manganese binding site 4 out of 4 in 4ut3
Go back to
Manganese binding site 4 out
of 4 in the X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of X-Ray Structure of the Human PP1 Gamma Catalytic Subunit Treated with Hydrogen Peroxide within 5.0Å range:
|
Reference:
C.X.Santos,
A.D.Hafstad,
M.Beretta,
M.Zhang,
C.Molenaar,
J.Kopec,
D.Fotinou,
T.V.Murray,
A.M.Cobb,
D.Martin,
M.Zeh Silva,
N.Anilkumar,
K.Schroder,
C.M.Shanahan,
A.C.Brewer,
R.P.Brandes,
E.Blanc,
M.Parsons,
V.Belousov,
R.Cammack,
R.C.Hider,
R.A.Steiner,
A.M.Shah.
Targeted Redox Inhibition of Protein Phosphatase 1 By NOX4 Regulates EIF2ALPHA-Mediated Stress Signaling. Embo J. V. 35 319 2016.
ISSN: ISSN 0261-4189
PubMed: 26742780
DOI: 10.15252/EMBJ.201592394
Page generated: Sat Oct 5 21:25:57 2024
ISSN: ISSN 0261-4189
PubMed: 26742780
DOI: 10.15252/EMBJ.201592394
Last articles
F in 7LI5F in 7LHZ
F in 7LH7
F in 7LDE
F in 7LEP
F in 7LDD
F in 7LGX
F in 7LGK
F in 7LG8
F in 7LD3