Manganese »
PDB 4k3v-4lt5 »
4lil »
Manganese in PDB 4lil: Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn
Protein crystallography data
The structure of Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn, PDB code: 4lil
was solved by
S.Vaithiyalingam,
B.F.Eichman,
W.J.Chazin,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 44.73 / 2.60 |
| Space group | P 43 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 79.346, 79.346, 148.200, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.4 / 24.8 |
Other elements in 4lil:
The structure of Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Manganese Binding Sites:
The binding sites of Manganese atom in the Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn
(pdb code 4lil). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn, PDB code: 4lil:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn, PDB code: 4lil:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 4lil
Go back to
Manganese binding site 1 out
of 2 in the Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn
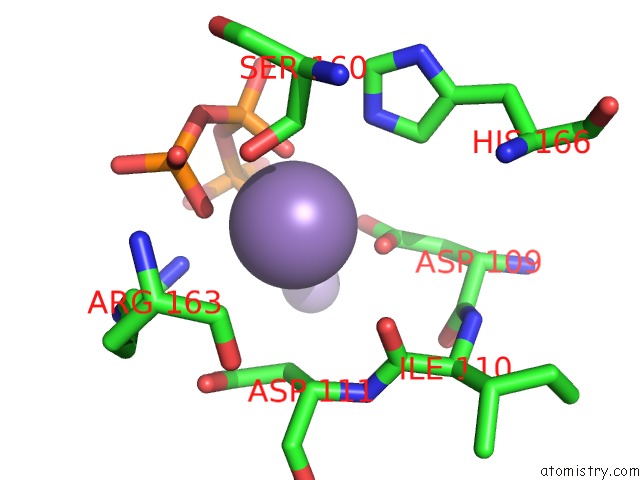
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn within 5.0Å range:
|
Manganese binding site 2 out of 2 in 4lil
Go back to
Manganese binding site 2 out
of 2 in the Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn
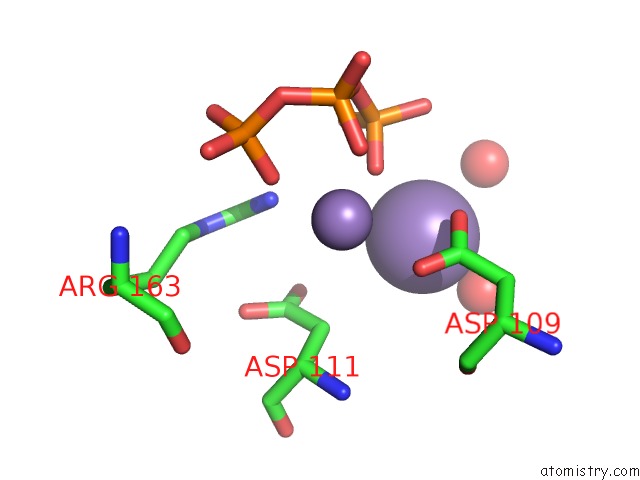
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Crystal Structure of the Catalytic Subunit of Human Primase Bound to Utp and Mn within 5.0Å range:
|
Reference:
S.Vaithiyalingam,
D.R.Arnett,
A.Aggarwal,
B.F.Eichman,
E.Fanning,
W.J.Chazin.
Insights Into Eukaryotic Primer Synthesis From Structures of the P48 Subunit of Human Dna Primase. J.Mol.Biol. V. 426 558 2014.
ISSN: ISSN 0022-2836
PubMed: 24239947
DOI: 10.1016/J.JMB.2013.11.007
Page generated: Sat Aug 16 14:29:22 2025
ISSN: ISSN 0022-2836
PubMed: 24239947
DOI: 10.1016/J.JMB.2013.11.007
Last articles
Mo in 2A9CMo in 2A9A
Mo in 2A9B
Mo in 2A99
Mo in 1ZXI
Mo in 1XDQ
Mo in 2A3P
Mo in 1Z13
Mo in 1Y5N
Mo in 1Y5L