Manganese »
PDB 4ima-4k28 »
4it2 »
Manganese in PDB 4it2: Mn(III)-Ppix Bound Tt H-Nox
Protein crystallography data
The structure of Mn(III)-Ppix Bound Tt H-Nox, PDB code: 4it2
was solved by
M.B.Winter,
P.J.Klemm,
C.M.Phillips-Piro,
K.M.Raymond,
M.A.Marletta,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 33.89 / 2.10 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 120.245, 45.416, 95.743, 90.00, 122.82, 90.00 |
| R / Rfree (%) | 20.9 / 24.5 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Mn(III)-Ppix Bound Tt H-Nox
(pdb code 4it2). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 3 binding sites of Manganese where determined in the Mn(III)-Ppix Bound Tt H-Nox, PDB code: 4it2:
Jump to Manganese binding site number: 1; 2; 3;
In total 3 binding sites of Manganese where determined in the Mn(III)-Ppix Bound Tt H-Nox, PDB code: 4it2:
Jump to Manganese binding site number: 1; 2; 3;
Manganese binding site 1 out of 3 in 4it2
Go back to
Manganese binding site 1 out
of 3 in the Mn(III)-Ppix Bound Tt H-Nox
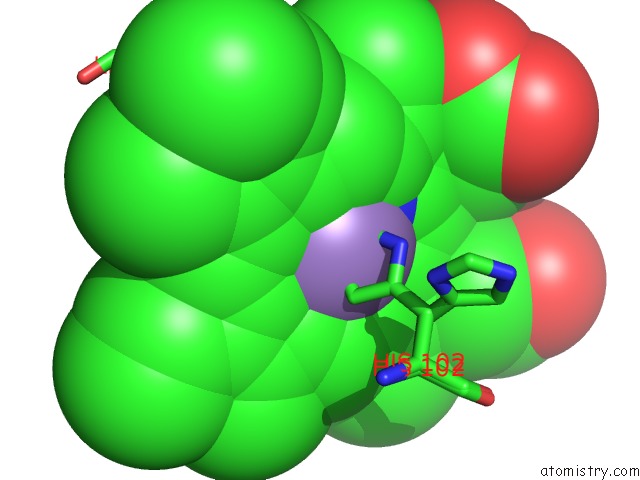
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Mn(III)-Ppix Bound Tt H-Nox within 5.0Å range:
|
Manganese binding site 2 out of 3 in 4it2
Go back to
Manganese binding site 2 out
of 3 in the Mn(III)-Ppix Bound Tt H-Nox
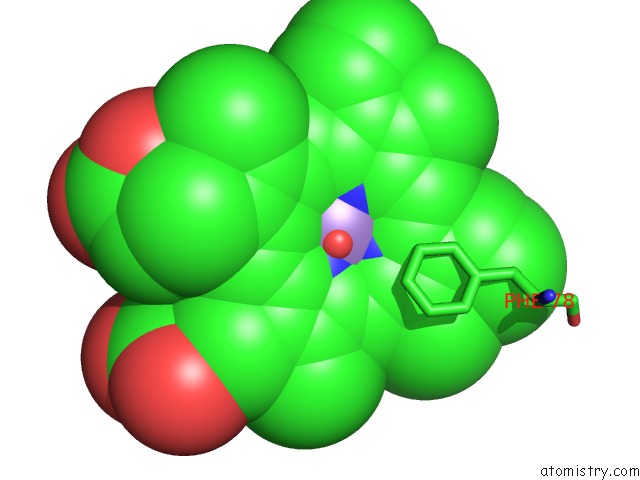
Mono view
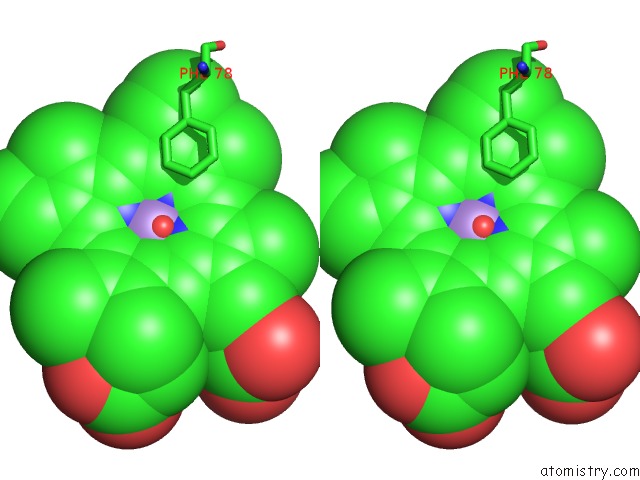
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Mn(III)-Ppix Bound Tt H-Nox within 5.0Å range:
|
Manganese binding site 3 out of 3 in 4it2
Go back to
Manganese binding site 3 out
of 3 in the Mn(III)-Ppix Bound Tt H-Nox

Mono view
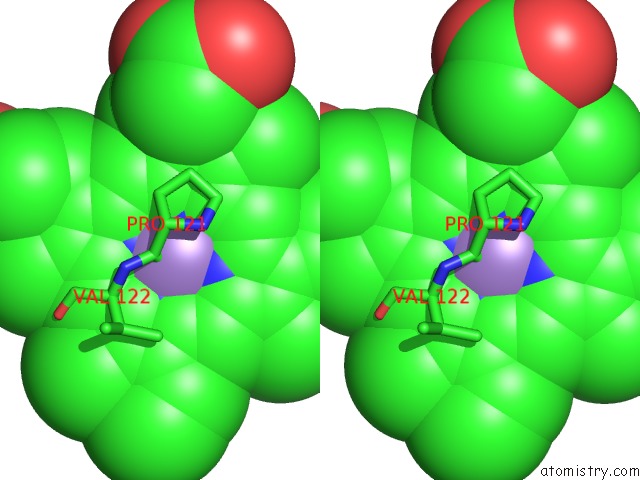
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Mn(III)-Ppix Bound Tt H-Nox within 5.0Å range:
|
Reference:
M.B.Winter,
P.J.Klemm,
C.M.Phillips-Piro,
K.N.Raymond,
M.A.Marletta.
Porphyrin-Substituted H-Nox Proteins As High-Relaxivity Mri Contrast Agents. Inorg.Chem. V. 52 2277 2013.
ISSN: ISSN 0020-1669
PubMed: 23394479
DOI: 10.1021/IC302685H
Page generated: Sat Aug 16 14:19:50 2025
ISSN: ISSN 0020-1669
PubMed: 23394479
DOI: 10.1021/IC302685H
Last articles
Ni in 5Q78Ni in 5Q79
Ni in 5Q77
Ni in 5Q76
Ni in 5Q75
Ni in 5Q72
Ni in 5Q73
Ni in 5Q74
Ni in 5Q70
Ni in 5Q6Y