Manganese »
PDB 4ima-4k28 »
4ima »
Manganese in PDB 4ima: The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp
Enzymatic activity of The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp
All present enzymatic activity of The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp:
2.7.1.40;
2.7.1.40;
Protein crystallography data
The structure of The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp, PDB code: 4ima
was solved by
B.Zhang,
T.Holyoak,
A.W.Fenton,
Q.L.Tang,
C.B.Prasannan,
J.P.Deng,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.89 / 1.95 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 82.751, 204.727, 86.532, 90.00, 96.75, 90.00 |
| R / Rfree (%) | 19.8 / 23.3 |
Manganese Binding Sites:
The binding sites of Manganese atom in the The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp
(pdb code 4ima). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 4 binding sites of Manganese where determined in the The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp, PDB code: 4ima:
Jump to Manganese binding site number: 1; 2; 3; 4;
In total 4 binding sites of Manganese where determined in the The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp, PDB code: 4ima:
Jump to Manganese binding site number: 1; 2; 3; 4;
Manganese binding site 1 out of 4 in 4ima
Go back to
Manganese binding site 1 out
of 4 in the The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp
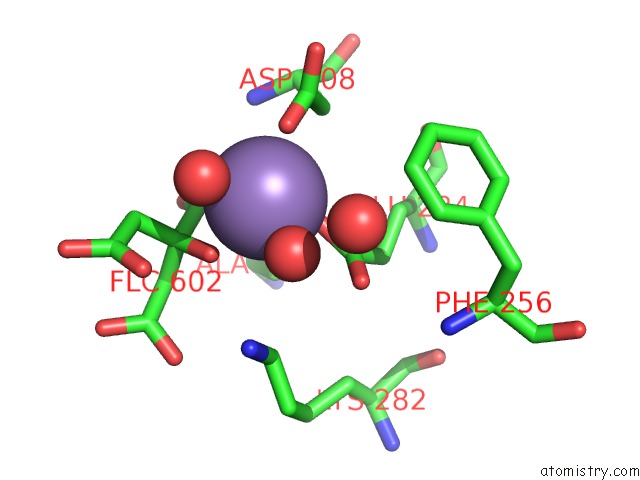
Mono view
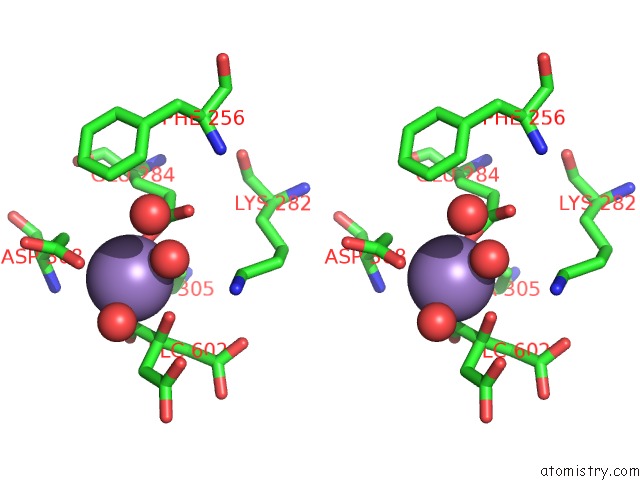
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp within 5.0Å range:
|
Manganese binding site 2 out of 4 in 4ima
Go back to
Manganese binding site 2 out
of 4 in the The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp
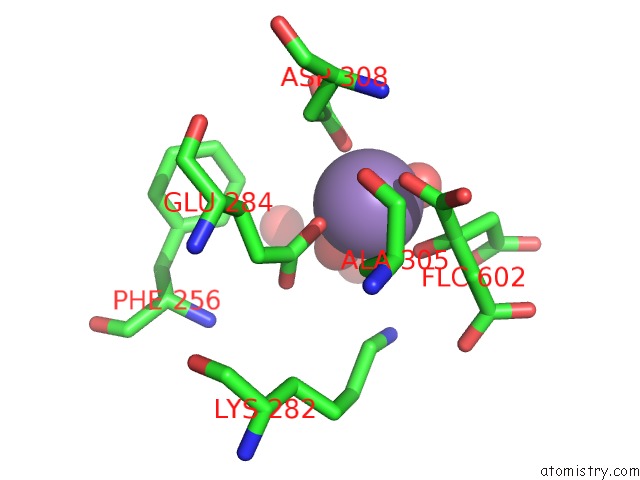
Mono view
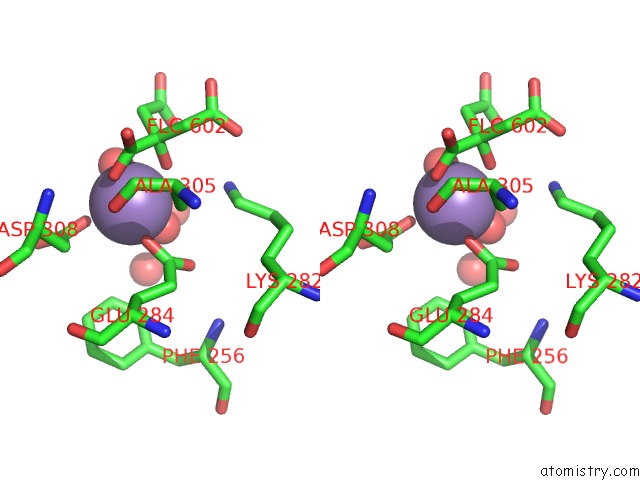
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp within 5.0Å range:
|
Manganese binding site 3 out of 4 in 4ima
Go back to
Manganese binding site 3 out
of 4 in the The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp
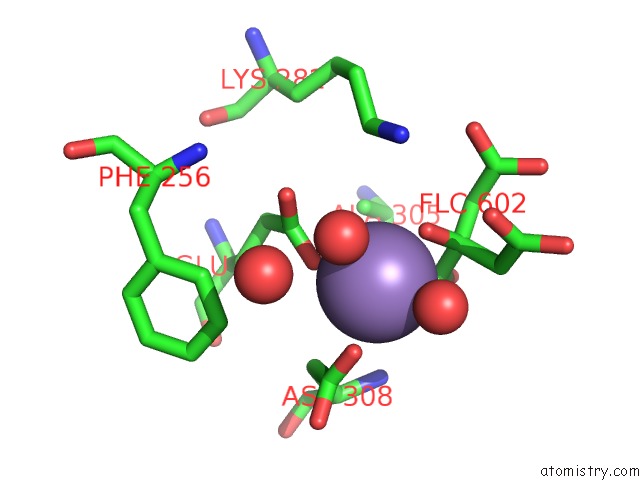
Mono view
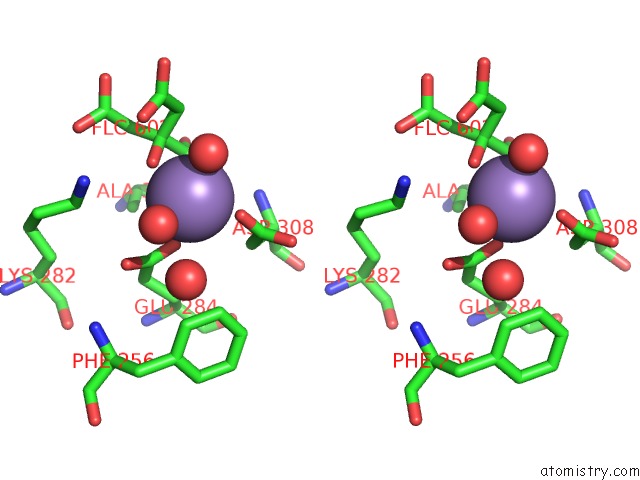
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp within 5.0Å range:
|
Manganese binding site 4 out of 4 in 4ima
Go back to
Manganese binding site 4 out
of 4 in the The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp
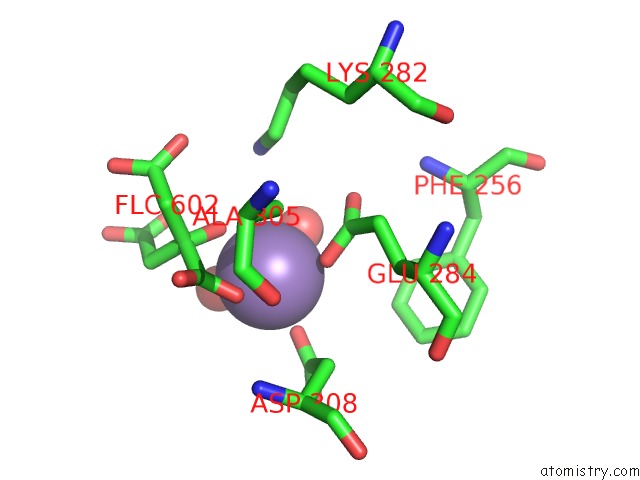
Mono view
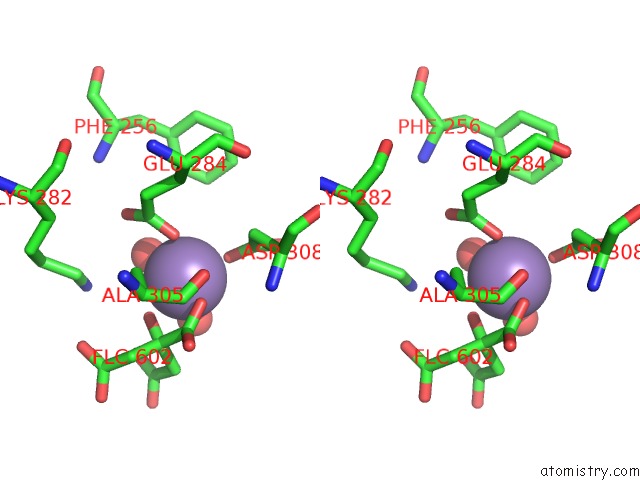
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of The Structure of C436M-Hlpyk in Complex with Citrate/Mn/Atp/Fru-1,6-Bp within 5.0Å range:
|
Reference:
T.Holyoak,
B.Zhang,
J.Deng,
Q.Tang,
C.B.Prasannan,
A.W.Fenton.
Energetic Coupling Between An Oxidizable Cysteine and the Phosphorylatable N-Terminus of Human Liver Pyruvate Kinase. Biochemistry V. 52 466 2013.
ISSN: ISSN 0006-2960
PubMed: 23270483
DOI: 10.1021/BI301341R
Page generated: Sat Oct 5 19:51:57 2024
ISSN: ISSN 0006-2960
PubMed: 23270483
DOI: 10.1021/BI301341R
Last articles
Ca in 5UJKCa in 5UJ6
Ca in 5UJP
Ca in 5UG7
Ca in 5UII
Ca in 5UGO
Ca in 5UFQ
Ca in 5UE4
Ca in 5UE5
Ca in 5UFE