Manganese »
PDB 3g0z-3hvq »
3gfz »
Manganese in PDB 3gfz: Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex
Protein crystallography data
The structure of Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex, PDB code: 3gfz
was solved by
T.Barends,
E.Hartmann,
J.Griese,
T.Beitlich,
N.Kirienko,
D.Ryjenkov,
J.Reinstein,
R.Shoeman,
M.Gomelsky,
I.Schlichting,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.54 / 2.05 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 68.450, 97.090, 127.170, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22.9 / 27.2 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex
(pdb code 3gfz). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 4 binding sites of Manganese where determined in the Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex, PDB code: 3gfz:
Jump to Manganese binding site number: 1; 2; 3; 4;
In total 4 binding sites of Manganese where determined in the Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex, PDB code: 3gfz:
Jump to Manganese binding site number: 1; 2; 3; 4;
Manganese binding site 1 out of 4 in 3gfz
Go back to
Manganese binding site 1 out
of 4 in the Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex
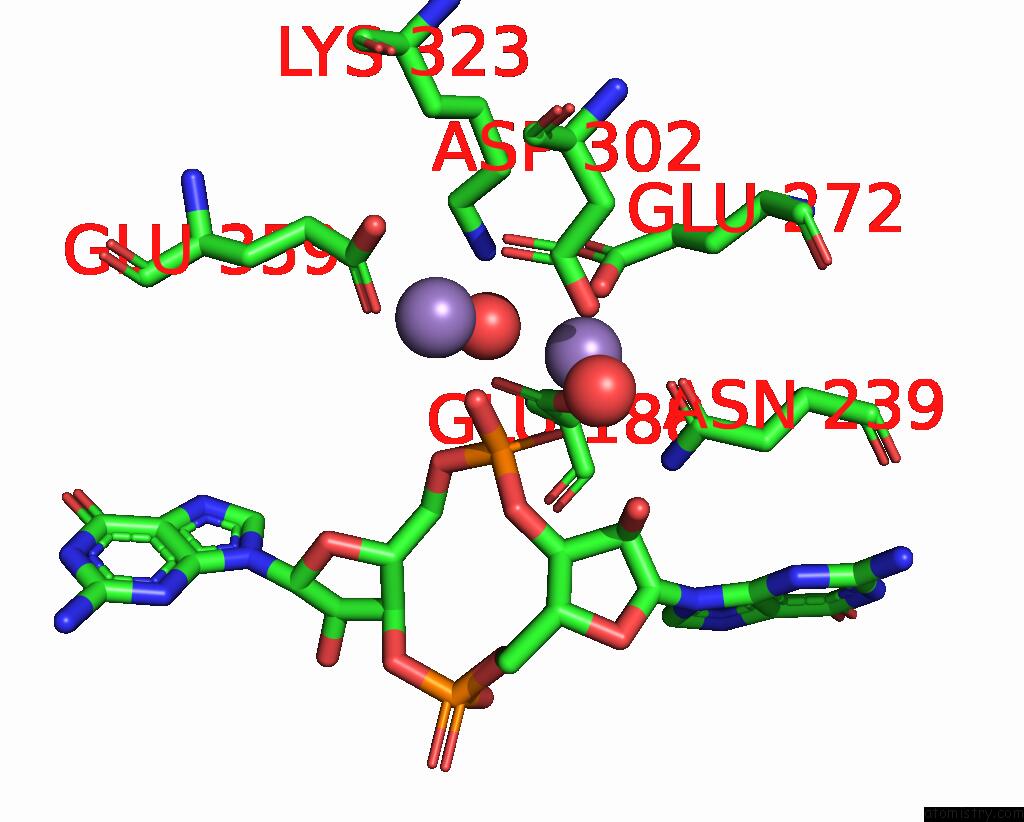
Mono view
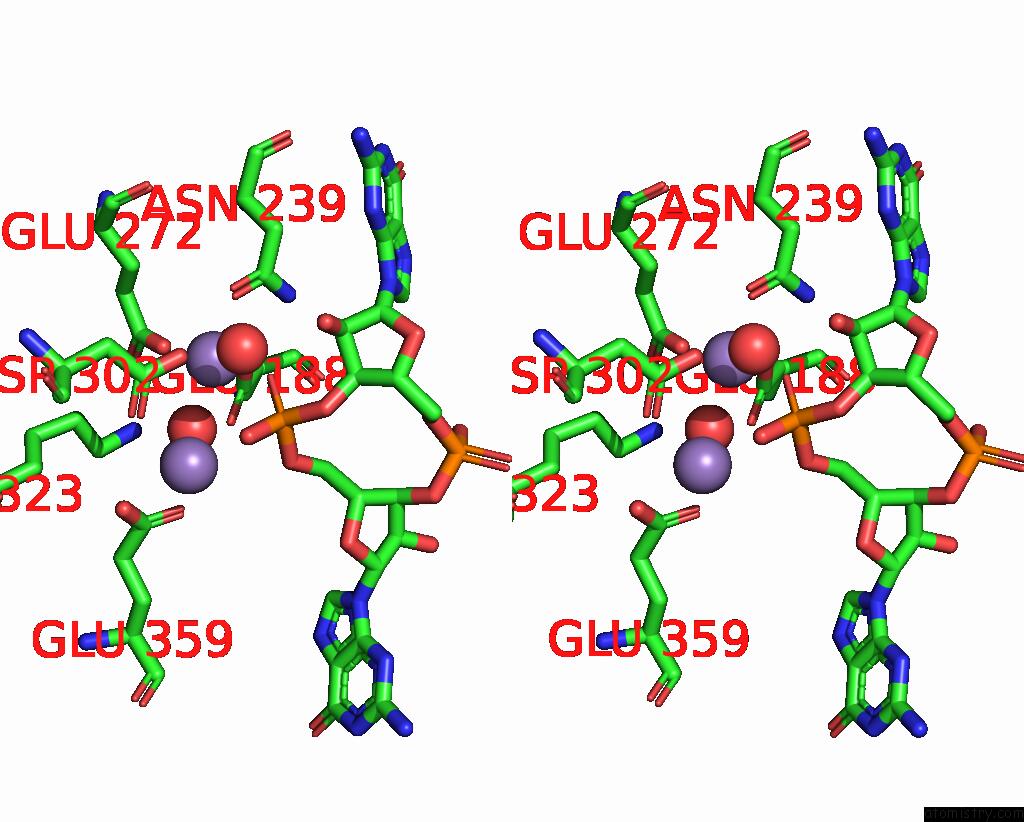
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex within 5.0Å range:
|
Manganese binding site 2 out of 4 in 3gfz
Go back to
Manganese binding site 2 out
of 4 in the Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex
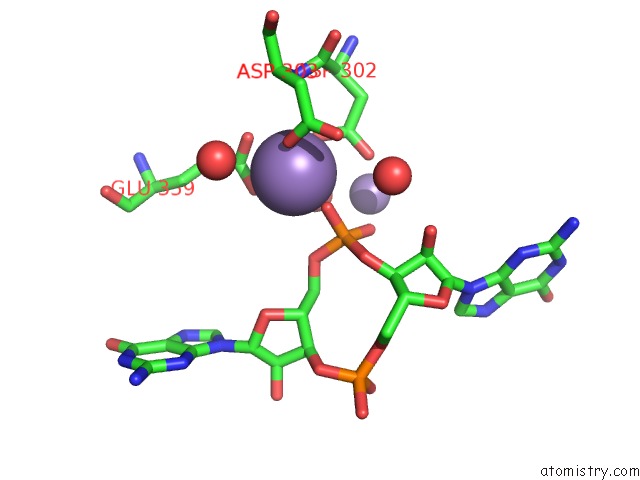
Mono view
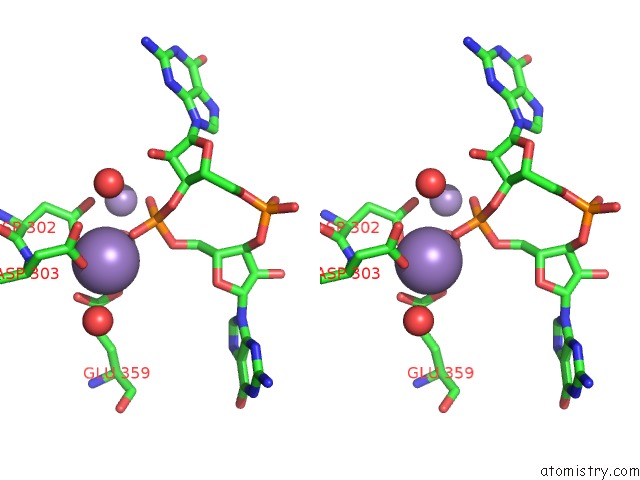
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex within 5.0Å range:
|
Manganese binding site 3 out of 4 in 3gfz
Go back to
Manganese binding site 3 out
of 4 in the Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex
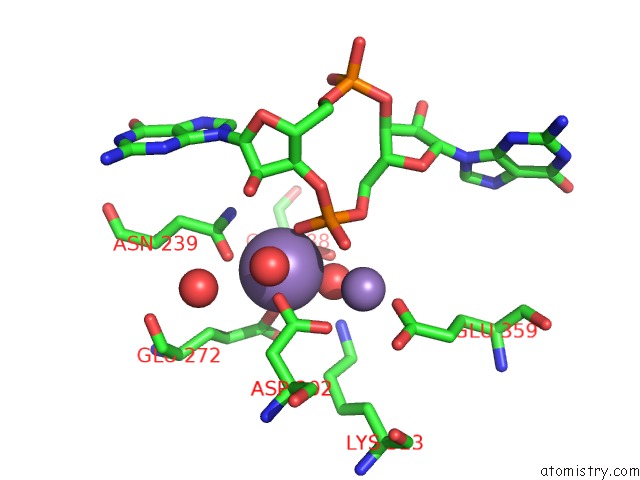
Mono view
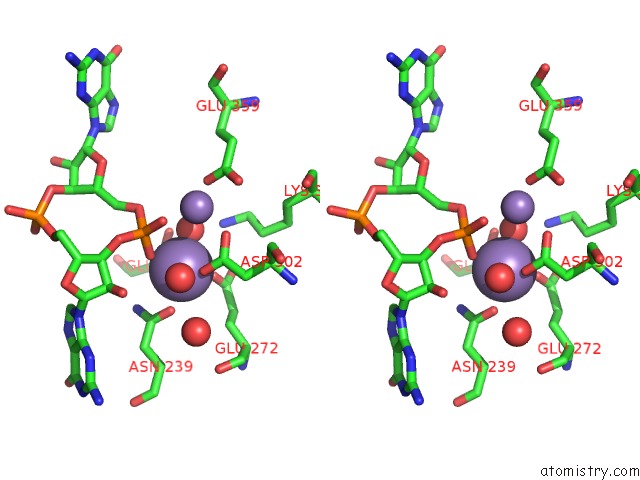
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex within 5.0Å range:
|
Manganese binding site 4 out of 4 in 3gfz
Go back to
Manganese binding site 4 out
of 4 in the Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex
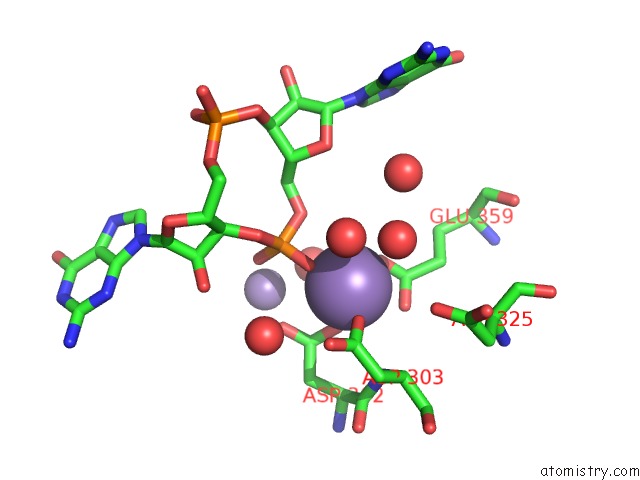
Mono view
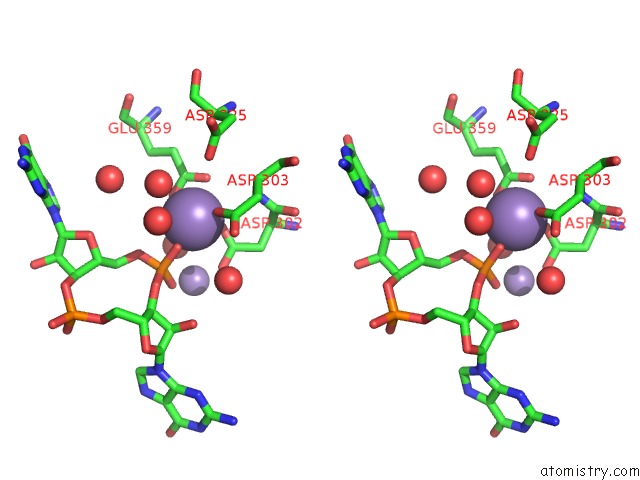
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of Klebsiella Pneumoniae BLRP1 pH 6 Manganese/Cy-Digmp Complex within 5.0Å range:
|
Reference:
T.R.Barends,
E.Hartmann,
J.J.Griese,
T.Beitlich,
N.V.Kirienko,
D.A.Ryjenkov,
J.Reinstein,
R.L.Shoeman,
M.Gomelsky,
I.Schlichting.
Structure and Mechanism of A Bacterial Light-Regulated Cyclic Nucleotide Phosphodiesterase. Nature V. 459 1015 2009.
ISSN: ISSN 0028-0836
PubMed: 19536266
DOI: 10.1038/NATURE07966
Page generated: Sat Aug 16 11:49:03 2025
ISSN: ISSN 0028-0836
PubMed: 19536266
DOI: 10.1038/NATURE07966
Last articles
Na in 1C1WNa in 1C1D
Na in 1C1S
Na in 1C1V
Na in 1C1U
Na in 1BUN
Na in 1BW9
Na in 1C10
Na in 1BG4
Na in 1BTK