Manganese »
PDB 2hvh-2jcj »
2hvh »
Manganese in PDB 2hvh: Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position)
Enzymatic activity of Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position)
All present enzymatic activity of Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position):
2.7.7.7;
2.7.7.7;
Protein crystallography data
The structure of Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position), PDB code: 2hvh
was solved by
J.J.Warren,
L.J.Forsberg,
L.S.Beese,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.30 / 2.49 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 94.060, 109.390, 151.310, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.1 / 26.2 |
Manganese Binding Sites:
The binding sites of Manganese atom in the Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position)
(pdb code 2hvh). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position), PDB code: 2hvh:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position), PDB code: 2hvh:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 2hvh
Go back to
Manganese binding site 1 out
of 2 in the Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position)
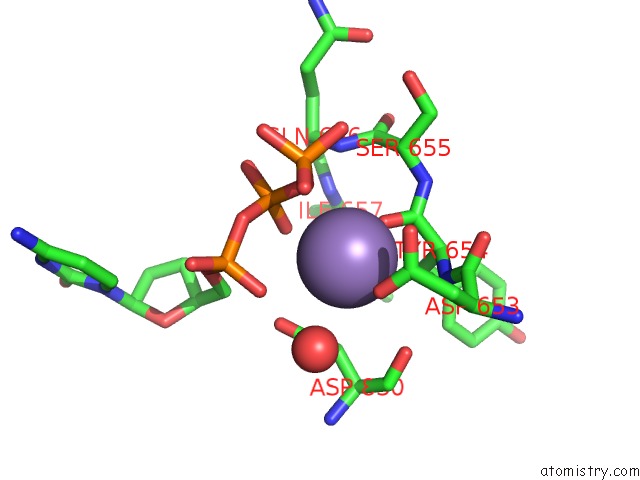
Mono view
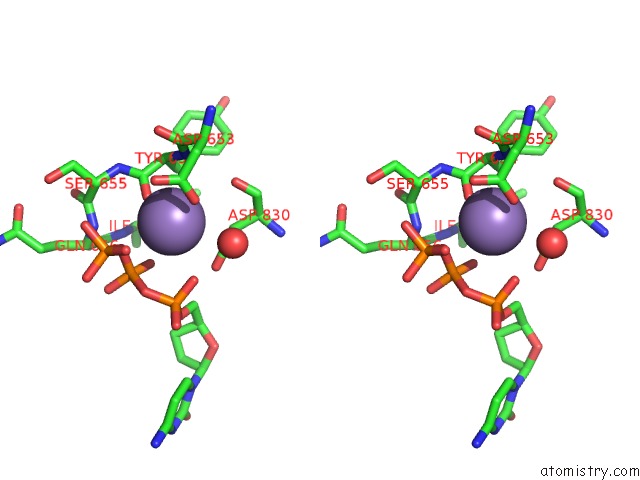
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position) within 5.0Å range:
|
Manganese binding site 2 out of 2 in 2hvh
Go back to
Manganese binding site 2 out
of 2 in the Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position)
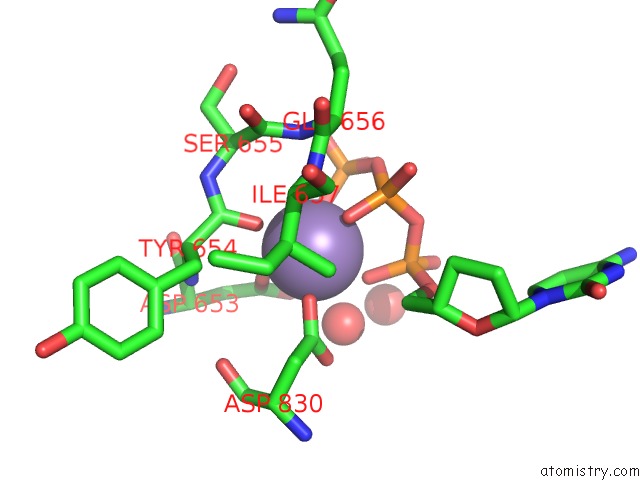
Mono view
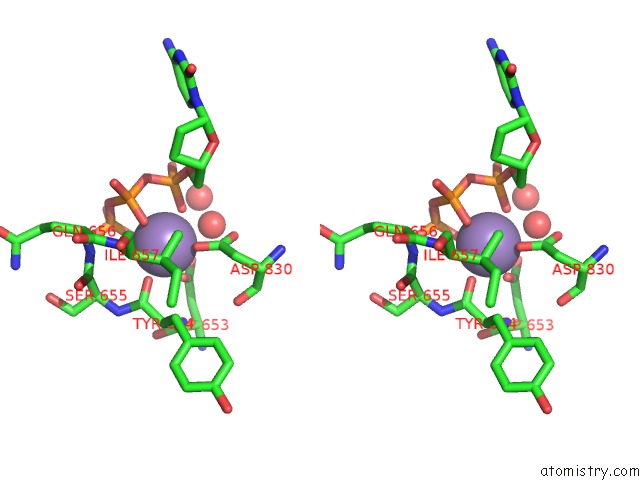
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of Ddctp:O6MEG Pair in the Polymerase Active Site (0 Position) within 5.0Å range:
|
Reference:
J.J.Warren,
L.J.Forsberg,
L.S.Beese.
The Structural Basis For the Mutagenicity of O6-Methyl-Guanine Lesions. Proc.Natl.Acad.Sci.Usa V. 103 19701 2006.
ISSN: ISSN 0027-8424
PubMed: 17179038
DOI: 10.1073/PNAS.0609580103
Page generated: Sat Oct 5 14:25:39 2024
ISSN: ISSN 0027-8424
PubMed: 17179038
DOI: 10.1073/PNAS.0609580103
Last articles
F in 7MEWF in 7ME8
F in 7MCF
F in 7MDP
F in 7M8V
F in 7MCK
F in 7MAZ
F in 7MBO
F in 7MCE
F in 7MB2