Manganese »
PDB 2ayl-2ce4 »
2bwy »
Manganese in PDB 2bwy: GLU383ALA Escherichia Coli Aminopeptidase P
Enzymatic activity of GLU383ALA Escherichia Coli Aminopeptidase P
All present enzymatic activity of GLU383ALA Escherichia Coli Aminopeptidase P:
3.4.11.9;
3.4.11.9;
Protein crystallography data
The structure of GLU383ALA Escherichia Coli Aminopeptidase P, PDB code: 2bwy
was solved by
S.C.Graham,
J.M.Guss,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 119.52 / 2.40 |
| Space group | I 41 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 138.578, 138.578, 231.389, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.2 / 20.7 |
Other elements in 2bwy:
The structure of GLU383ALA Escherichia Coli Aminopeptidase P also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
Manganese Binding Sites:
The binding sites of Manganese atom in the GLU383ALA Escherichia Coli Aminopeptidase P
(pdb code 2bwy). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the GLU383ALA Escherichia Coli Aminopeptidase P, PDB code: 2bwy:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the GLU383ALA Escherichia Coli Aminopeptidase P, PDB code: 2bwy:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 2bwy
Go back to
Manganese binding site 1 out
of 2 in the GLU383ALA Escherichia Coli Aminopeptidase P
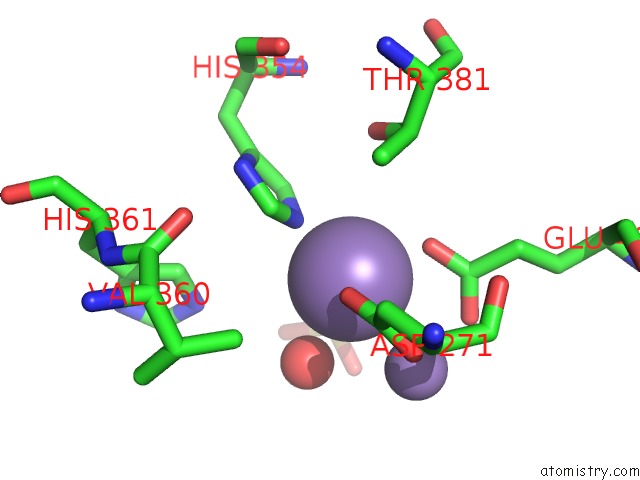
Mono view
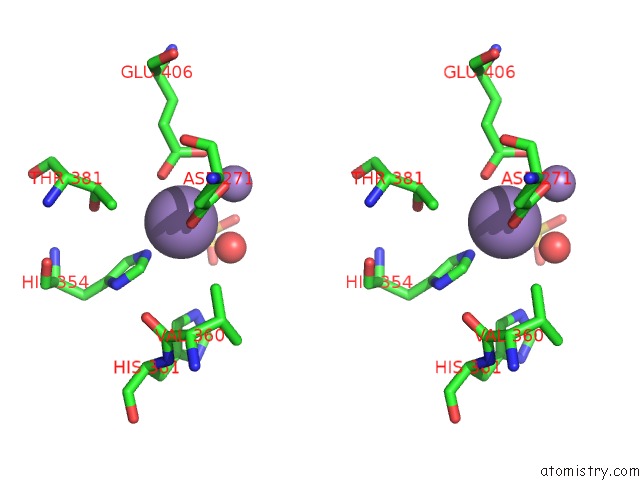
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of GLU383ALA Escherichia Coli Aminopeptidase P within 5.0Å range:
|
Manganese binding site 2 out of 2 in 2bwy
Go back to
Manganese binding site 2 out
of 2 in the GLU383ALA Escherichia Coli Aminopeptidase P
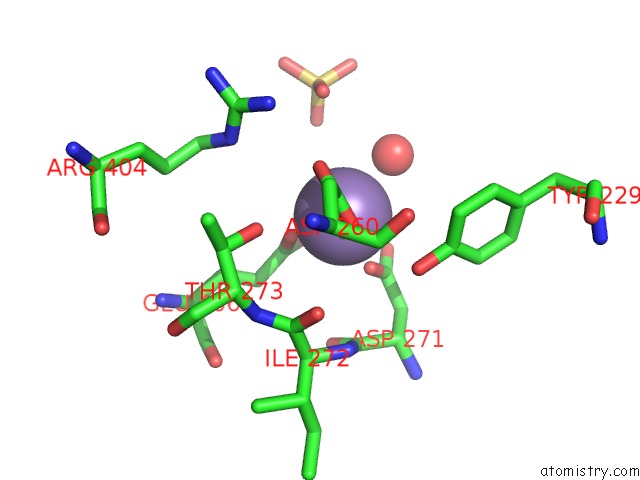
Mono view
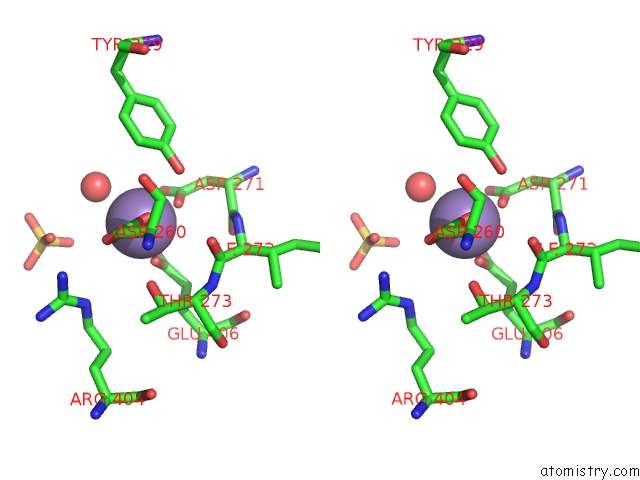
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of GLU383ALA Escherichia Coli Aminopeptidase P within 5.0Å range:
|
Reference:
S.C.Graham,
P.E.Lilley,
M.Lee,
P.M.Schaeffer,
A.V.Kralicek,
N.E.Dixon,
J.M.Guss.
Kinetic and Crystallographic Analysis of Mutant Escherichia Coli Aminopeptidase P: Insights Into Substrate Recognition and the Mechanism of Catalysis. Biochemistry V. 45 964 2006.
ISSN: ISSN 0006-2960
PubMed: 16411772
DOI: 10.1021/BI0518904
Page generated: Sat Oct 5 13:37:02 2024
ISSN: ISSN 0006-2960
PubMed: 16411772
DOI: 10.1021/BI0518904
Last articles
Cl in 5VUSCl in 5VTK
Cl in 5VSV
Cl in 5VR0
Cl in 5VTI
Cl in 5VTD
Cl in 5VT7
Cl in 5VSK
Cl in 5VSB
Cl in 5VS7