Manganese »
PDB 1w2c-1xie »
1w4a »
Manganese in PDB 1w4a: P4 Protein From PHI12 in Complex with Ampcpp and Mn
Protein crystallography data
The structure of P4 Protein From PHI12 in Complex with Ampcpp and Mn, PDB code: 1w4a
was solved by
E.J.Mancini,
D.E.Kainov,
J.M.Grimes,
R.Tuma,
D.H.Bamford,
D.I.Stuart,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 2.40 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 105.600, 129.700, 159.300, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 24.3 / 18.9 |
Manganese Binding Sites:
The binding sites of Manganese atom in the P4 Protein From PHI12 in Complex with Ampcpp and Mn
(pdb code 1w4a). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 3 binding sites of Manganese where determined in the P4 Protein From PHI12 in Complex with Ampcpp and Mn, PDB code: 1w4a:
Jump to Manganese binding site number: 1; 2; 3;
In total 3 binding sites of Manganese where determined in the P4 Protein From PHI12 in Complex with Ampcpp and Mn, PDB code: 1w4a:
Jump to Manganese binding site number: 1; 2; 3;
Manganese binding site 1 out of 3 in 1w4a
Go back to
Manganese binding site 1 out
of 3 in the P4 Protein From PHI12 in Complex with Ampcpp and Mn
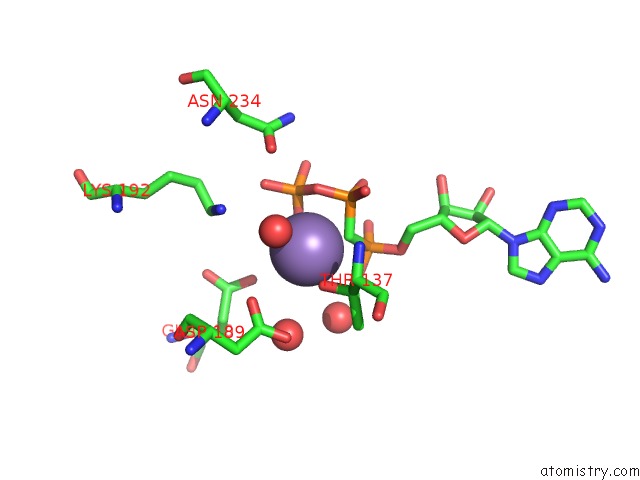
Mono view
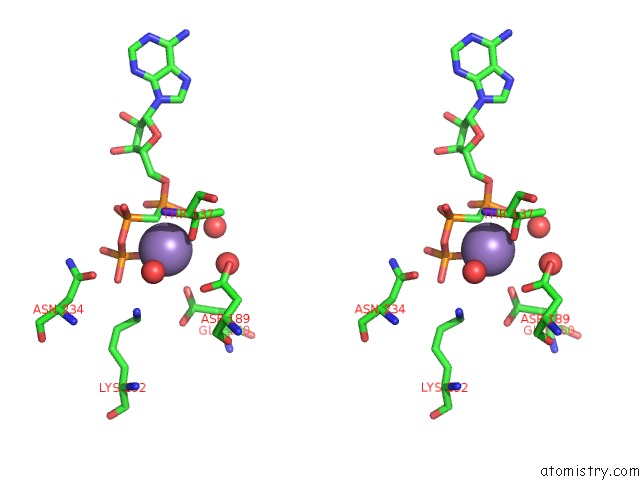
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of P4 Protein From PHI12 in Complex with Ampcpp and Mn within 5.0Å range:
|
Manganese binding site 2 out of 3 in 1w4a
Go back to
Manganese binding site 2 out
of 3 in the P4 Protein From PHI12 in Complex with Ampcpp and Mn
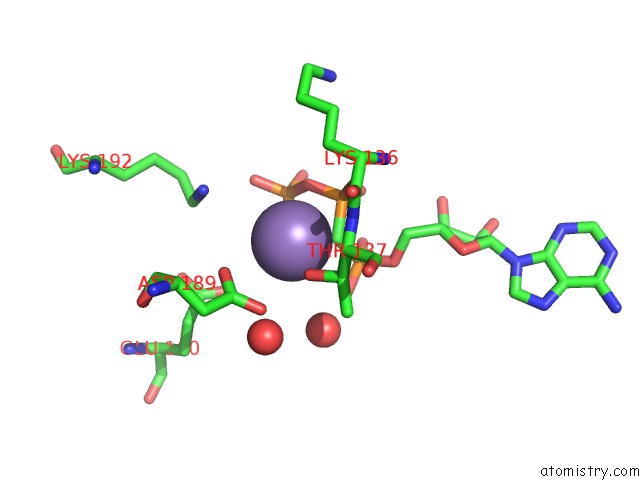
Mono view
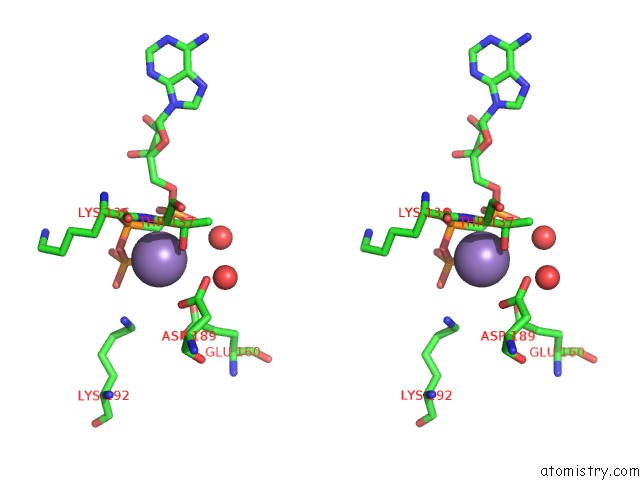
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of P4 Protein From PHI12 in Complex with Ampcpp and Mn within 5.0Å range:
|
Manganese binding site 3 out of 3 in 1w4a
Go back to
Manganese binding site 3 out
of 3 in the P4 Protein From PHI12 in Complex with Ampcpp and Mn
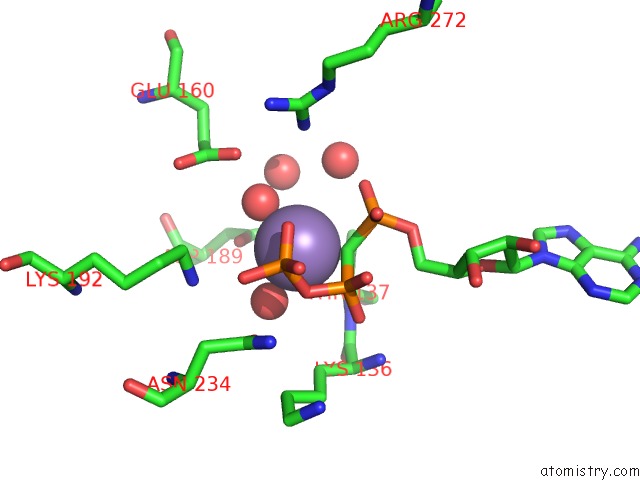
Mono view
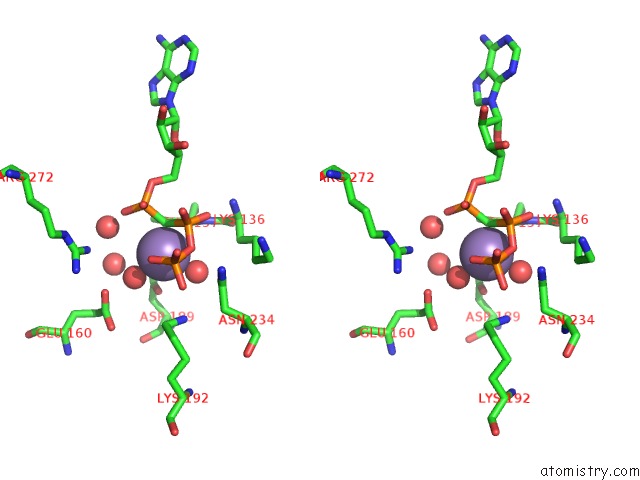
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of P4 Protein From PHI12 in Complex with Ampcpp and Mn within 5.0Å range:
|
Reference:
E.J.Mancini,
D.E.Kainov,
J.M.Grimes,
R.Tuma,
D.H.Bamford,
D.I.Stuart.
Atomic Snapshots of An Rna Packaging Motor Reveal Conformational Changes Linking Atp Hydrolysis to Rna Translocation Cell(Cambridge,Mass.) V. 118 743 2004.
ISSN: ISSN 0092-8674
PubMed: 15369673
DOI: 10.1016/J.CELL.2004.09.007
Page generated: Sat Oct 5 12:53:15 2024
ISSN: ISSN 0092-8674
PubMed: 15369673
DOI: 10.1016/J.CELL.2004.09.007
Last articles
Cl in 3TRGCl in 3TQR
Cl in 3TPZ
Cl in 3TOZ
Cl in 3TPR
Cl in 3TPL
Cl in 3TPJ
Cl in 3TPD
Cl in 3TPB
Cl in 3TO3