Manganese »
PDB 1n0n-1o99 »
1o98 »
Manganese in PDB 1o98: 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate
Enzymatic activity of 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate
All present enzymatic activity of 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate:
5.4.2.1;
5.4.2.1;
Protein crystallography data
The structure of 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate, PDB code: 1o98
was solved by
D.J.Rigden,
E.Lamani,
J.E.Littlejohn,
M.J.Jedrzejas,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 1.4 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.515, 205.787, 125.018, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19 / 19.8 |
Manganese Binding Sites:
The binding sites of Manganese atom in the 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate
(pdb code 1o98). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 2 binding sites of Manganese where determined in the 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate, PDB code: 1o98:
Jump to Manganese binding site number: 1; 2;
In total 2 binding sites of Manganese where determined in the 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate, PDB code: 1o98:
Jump to Manganese binding site number: 1; 2;
Manganese binding site 1 out of 2 in 1o98
Go back to
Manganese binding site 1 out
of 2 in the 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate
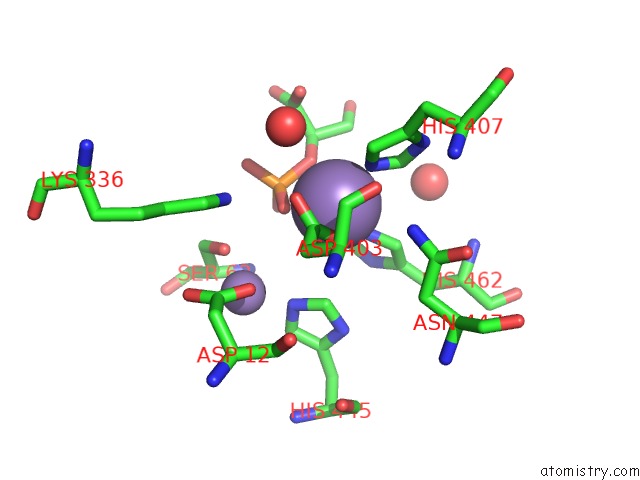
Mono view
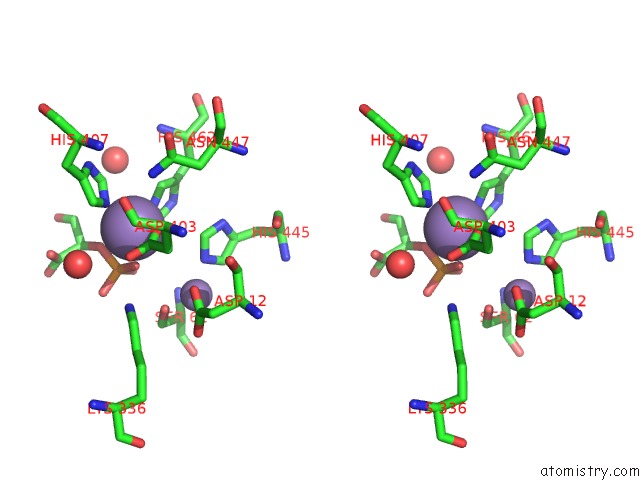
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate within 5.0Å range:
|
Manganese binding site 2 out of 2 in 1o98
Go back to
Manganese binding site 2 out
of 2 in the 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate
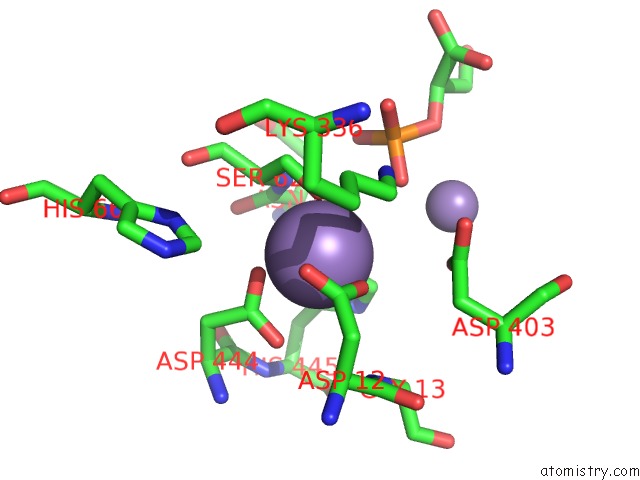
Mono view
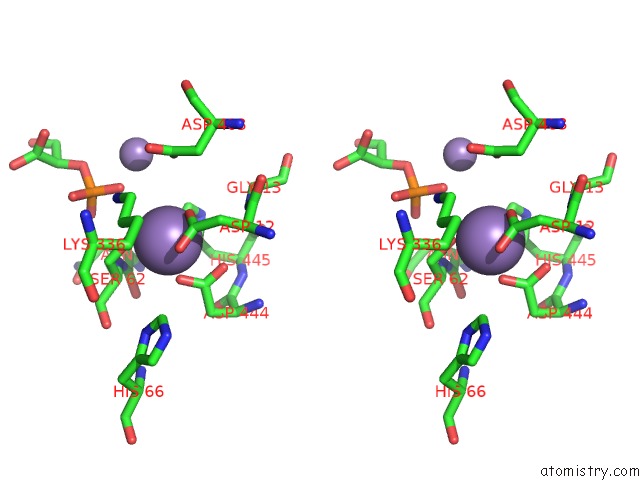
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of 1.4A Crystal Structure of Phosphoglycerate Mutase From Bacillus Stearothermophilus Complexed with 2-Phosphoglycerate within 5.0Å range:
|
Reference:
D.J.Rigden,
L.V.Mello,
E.Lamani,
J.E.Littlejohn,
M.J.Jedrzejas.
Insights Into the Catalytic Mechanism of Cofactor-Independent Phosphoglycerate Mutase From X-Ray Crystallography, Simulated Dynamics and Molecular Modeling J.Mol.Biol. V. 328 909 2003.
ISSN: ISSN 0022-2836
PubMed: 12729763
DOI: 10.1016/S0022-2836(03)00350-4
Page generated: Sat Aug 16 08:38:36 2025
ISSN: ISSN 0022-2836
PubMed: 12729763
DOI: 10.1016/S0022-2836(03)00350-4
Last articles
Na in 1Q86Na in 1QIO
Na in 1Q82
Na in 1QDR
Na in 1Q9I
Na in 1Q8C
Na in 1Q93
Na in 1Q81
Na in 1Q7Y
Na in 1PX4