Manganese »
PDB 1j54-1khb »
1kgp »
Manganese in PDB 1kgp: R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
Protein crystallography data
The structure of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form, PDB code: 1kgp
was solved by
M.Hogbom,
Y.Huque,
B.M.Sjoberg,
P.Nordlund,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.00 / 2.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 50.670, 91.196, 137.270, 90.00, 91.45, 90.00 |
| R / Rfree (%) | 17.7 / 23.6 |
Manganese Binding Sites:
The binding sites of Manganese atom in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
(pdb code 1kgp). This binding sites where shown within
5.0 Angstroms radius around Manganese atom.
In total 10 binding sites of Manganese where determined in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form, PDB code: 1kgp:
Jump to Manganese binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
In total 10 binding sites of Manganese where determined in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form, PDB code: 1kgp:
Jump to Manganese binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Manganese binding site 1 out of 10 in 1kgp
Go back to
Manganese binding site 1 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
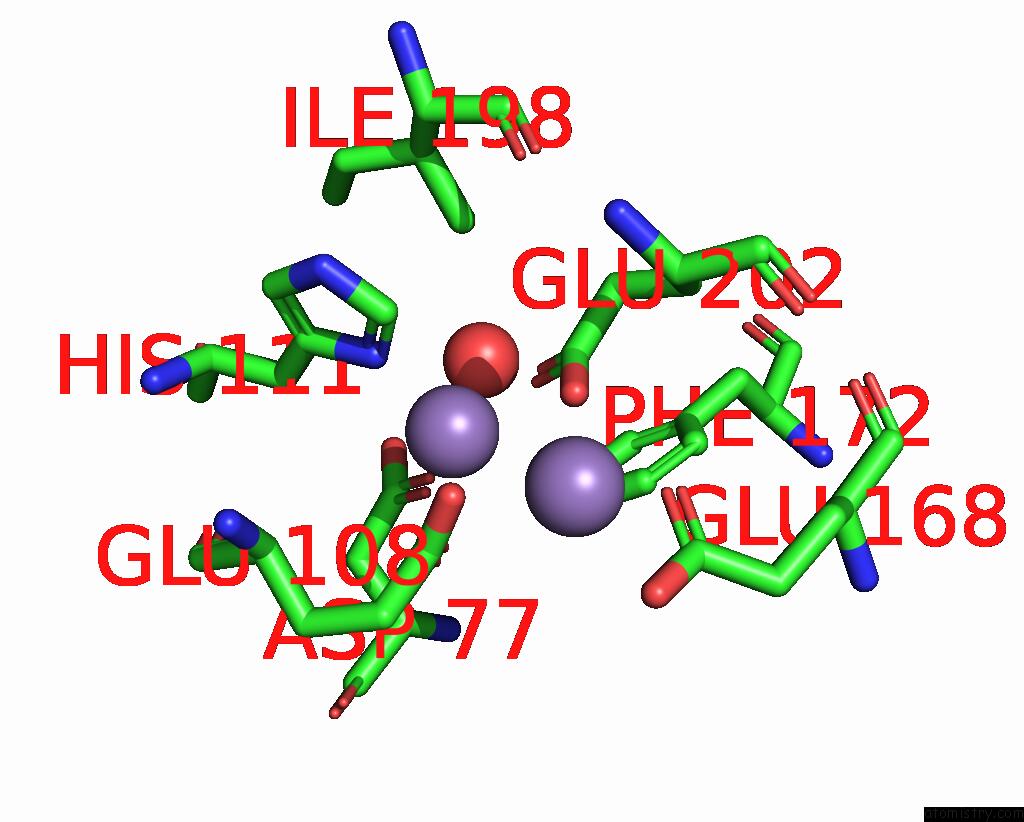
Mono view
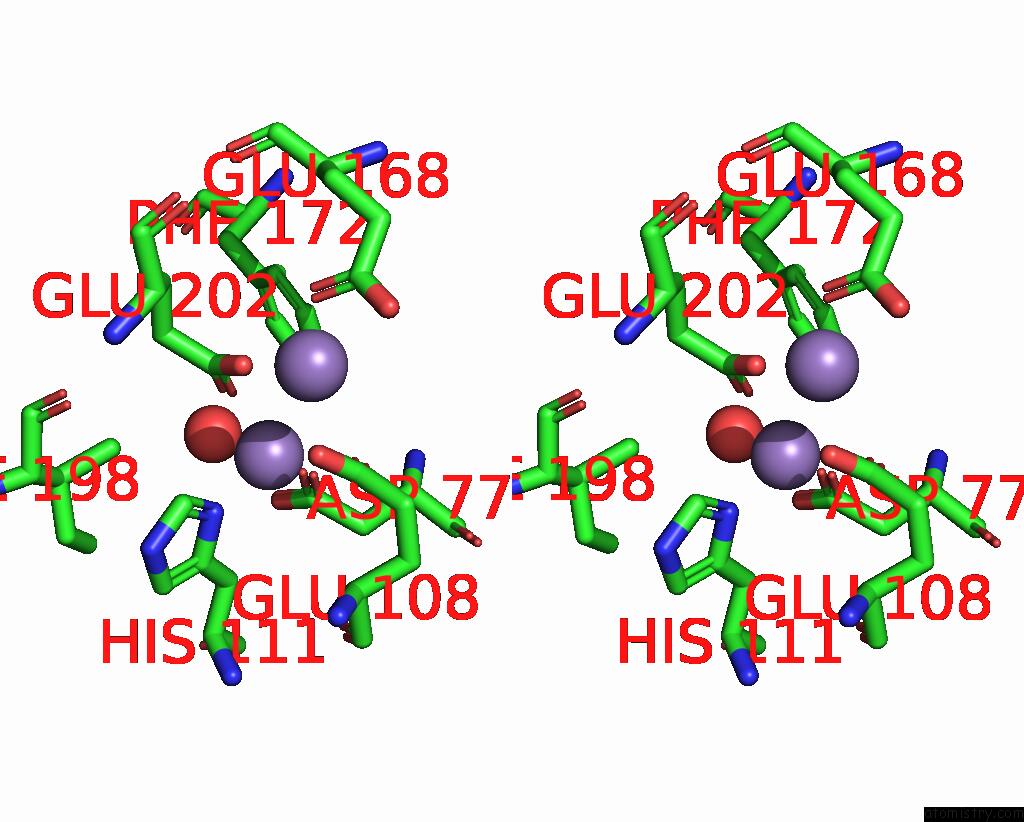
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 1 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 2 out of 10 in 1kgp
Go back to
Manganese binding site 2 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
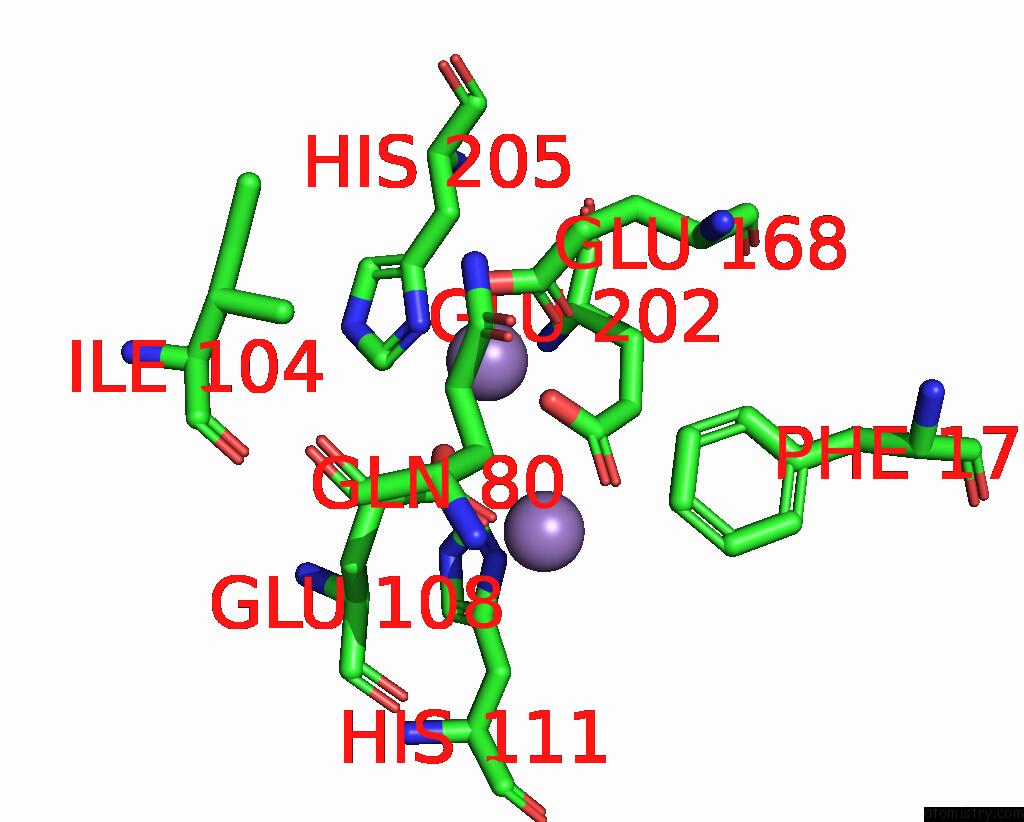
Mono view
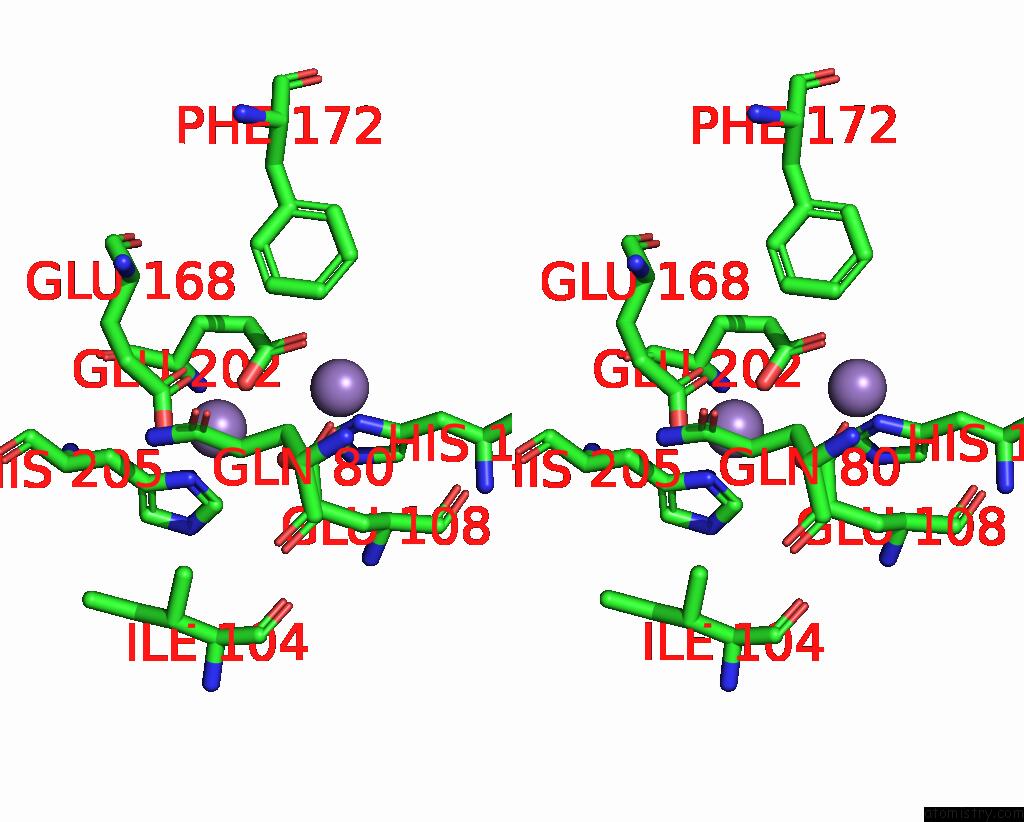
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 2 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 3 out of 10 in 1kgp
Go back to
Manganese binding site 3 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
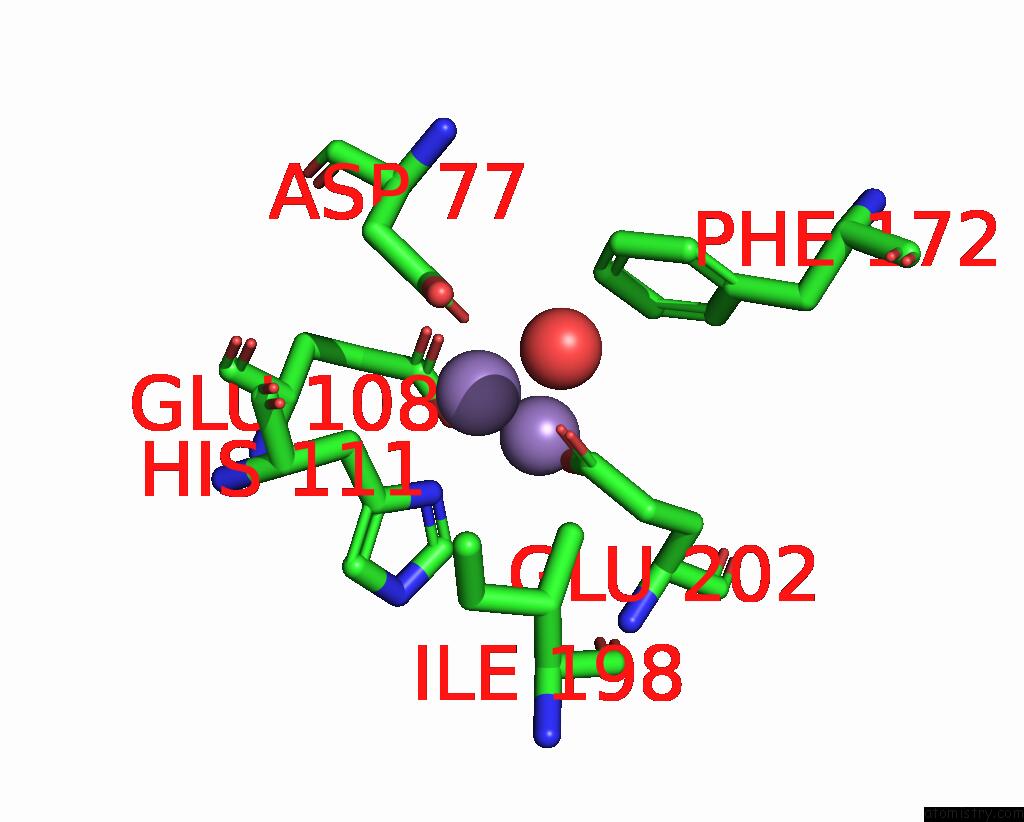
Mono view
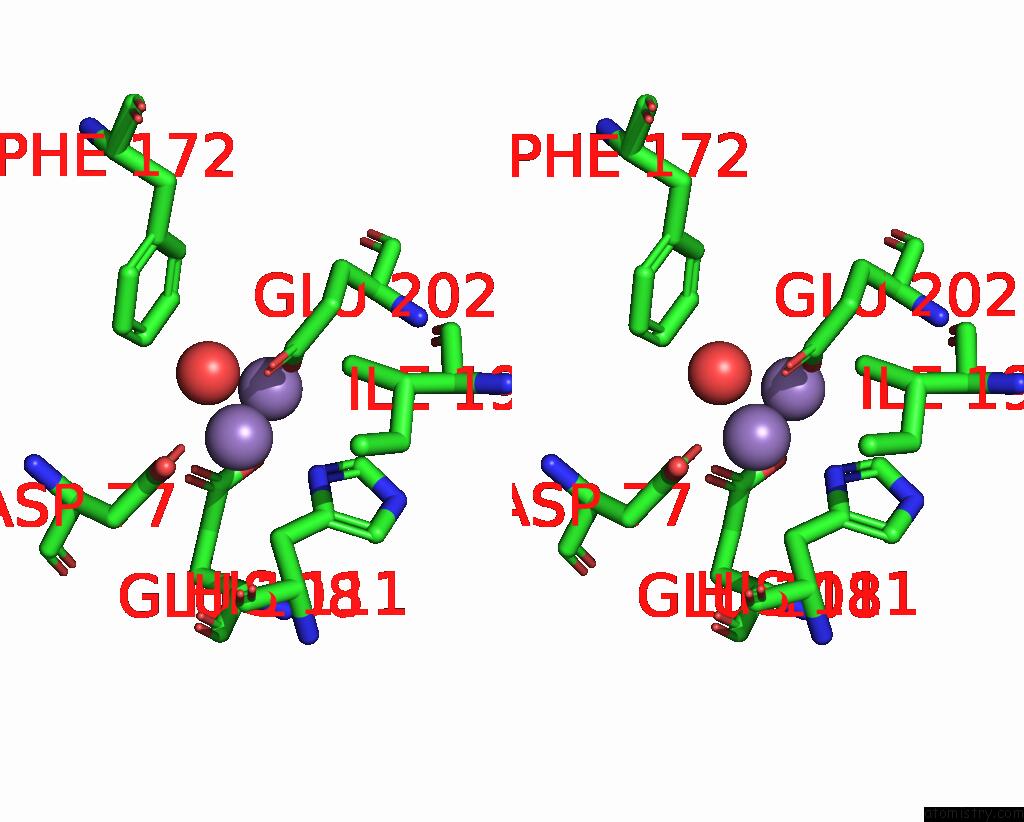
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 3 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 4 out of 10 in 1kgp
Go back to
Manganese binding site 4 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
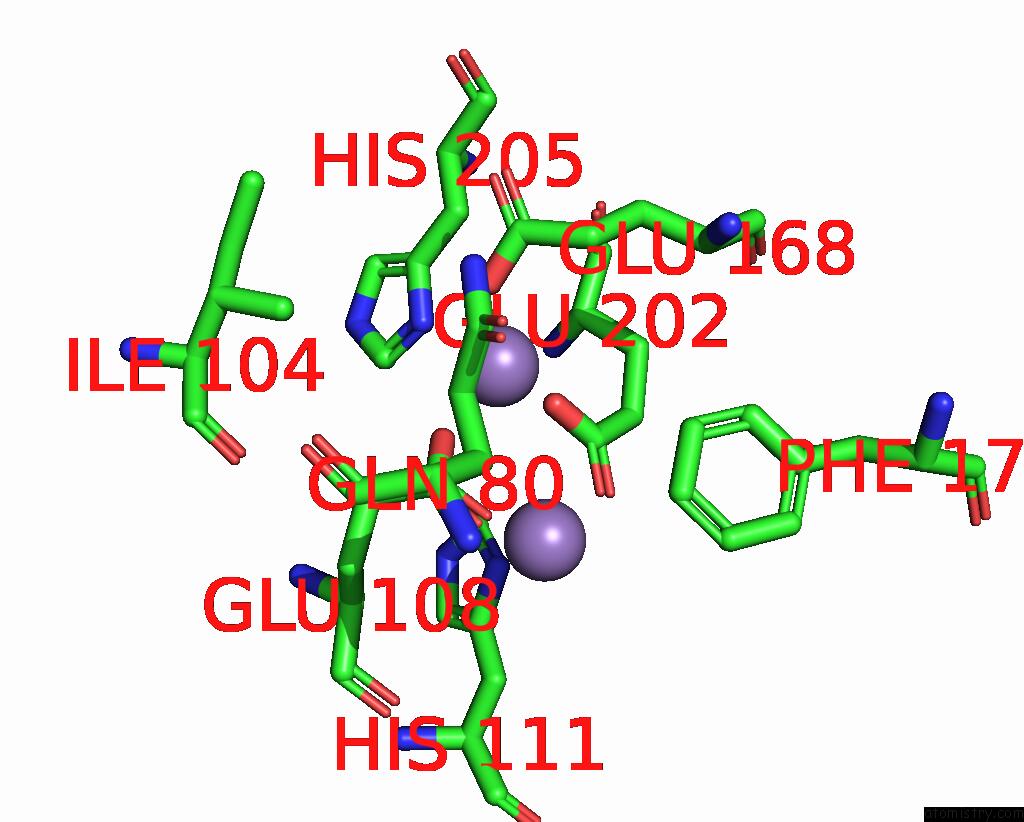
Mono view
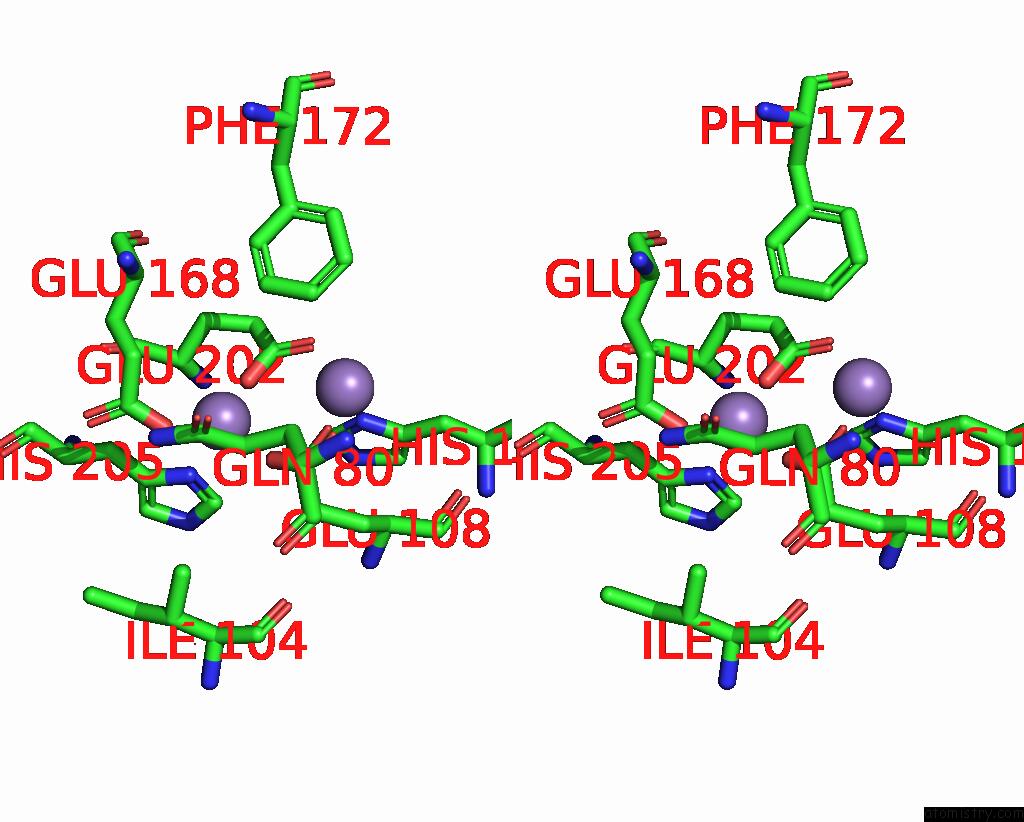
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 4 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 5 out of 10 in 1kgp
Go back to
Manganese binding site 5 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
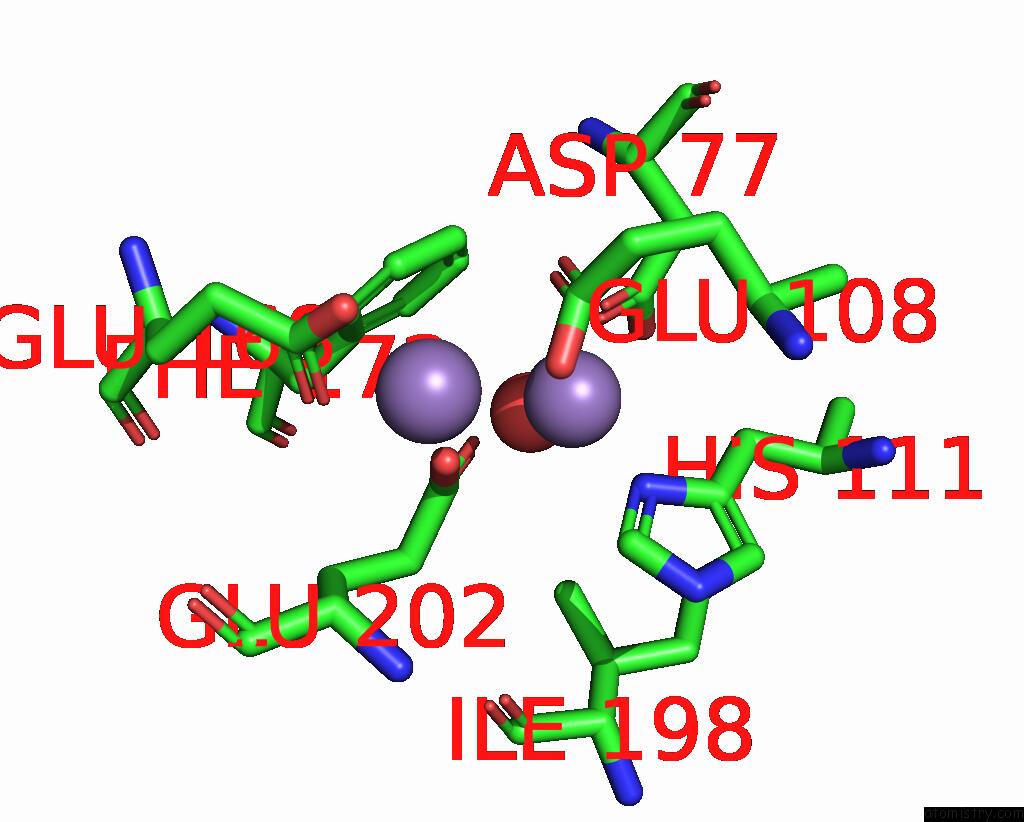
Mono view
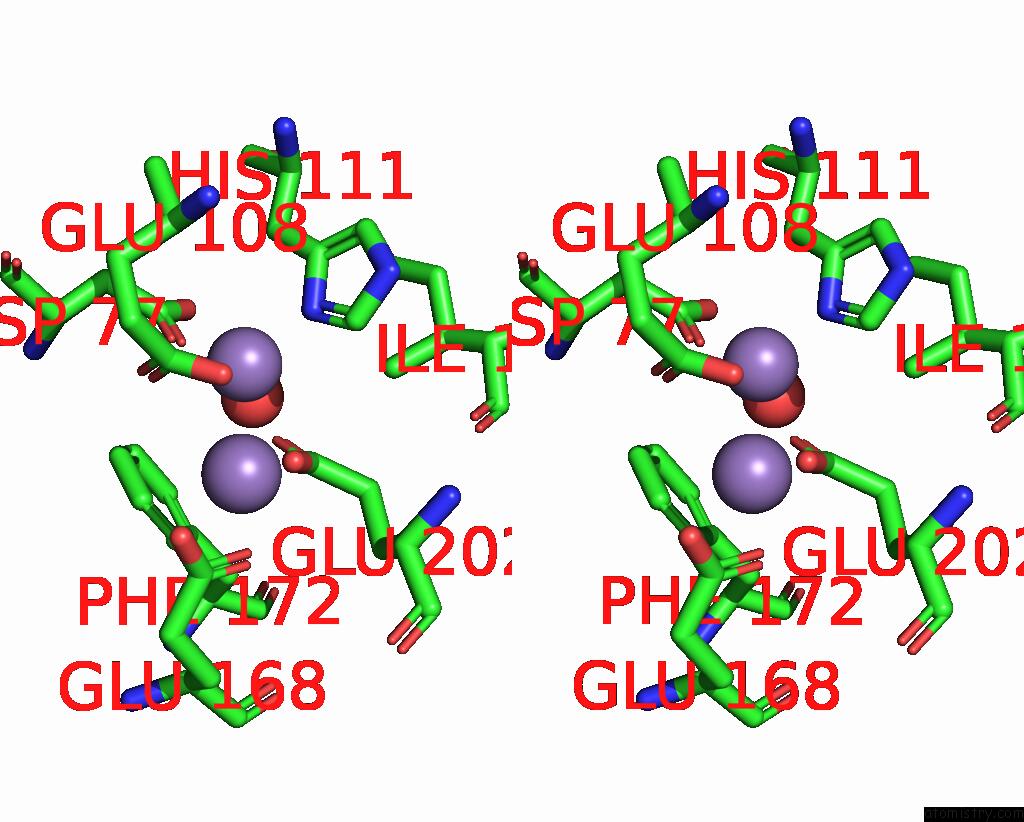
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 5 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 6 out of 10 in 1kgp
Go back to
Manganese binding site 6 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
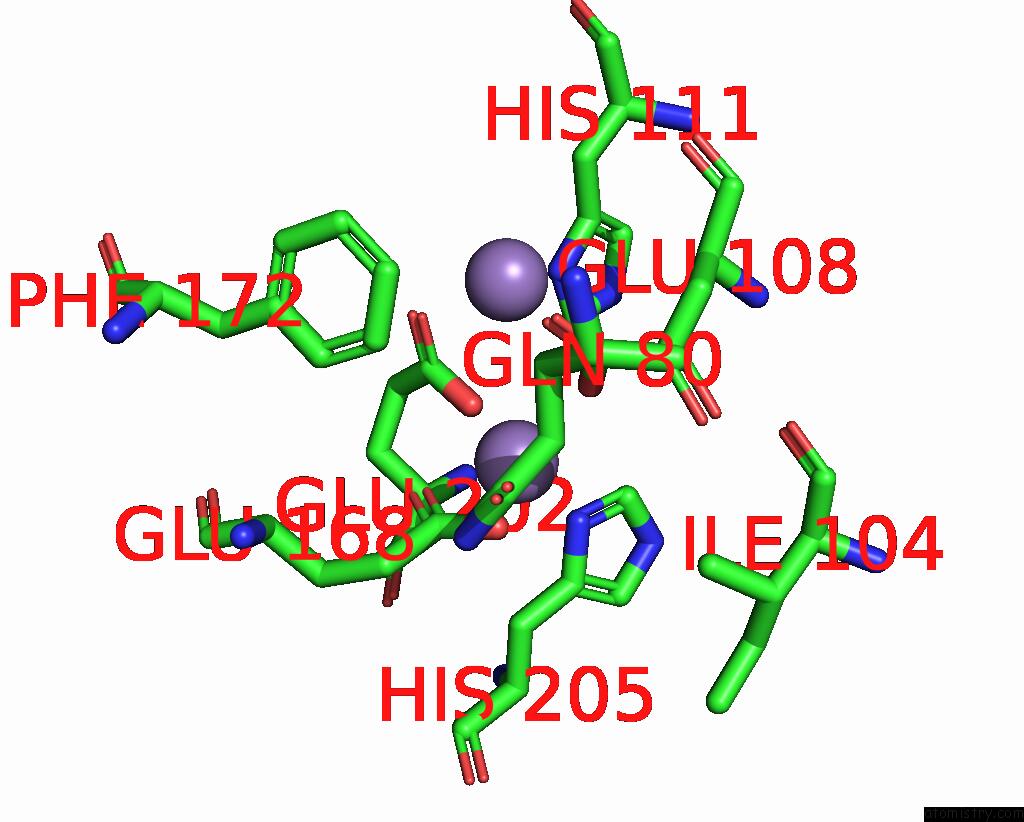
Mono view
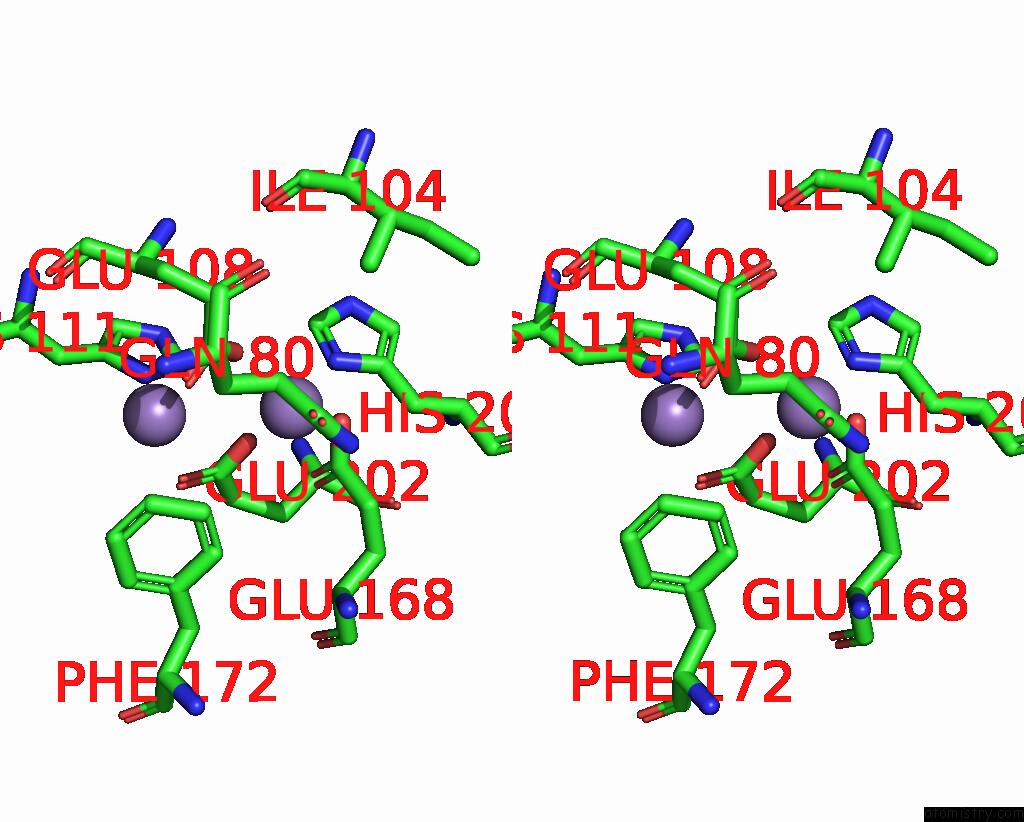
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 6 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 7 out of 10 in 1kgp
Go back to
Manganese binding site 7 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
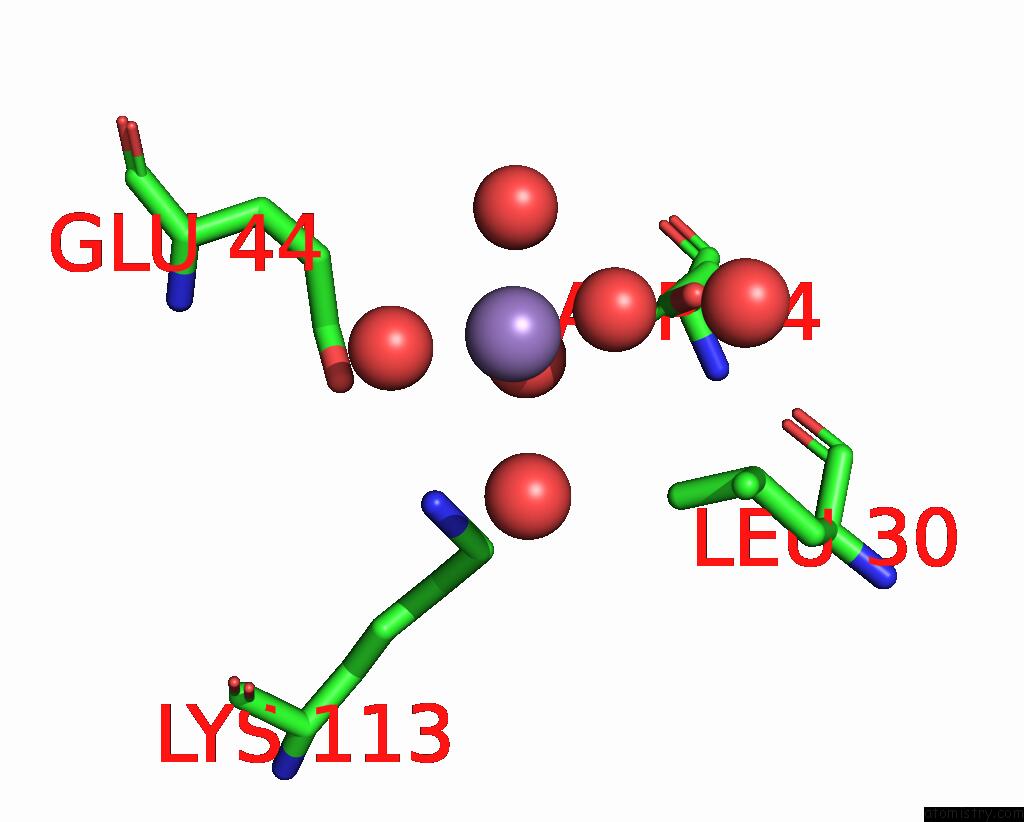
Mono view
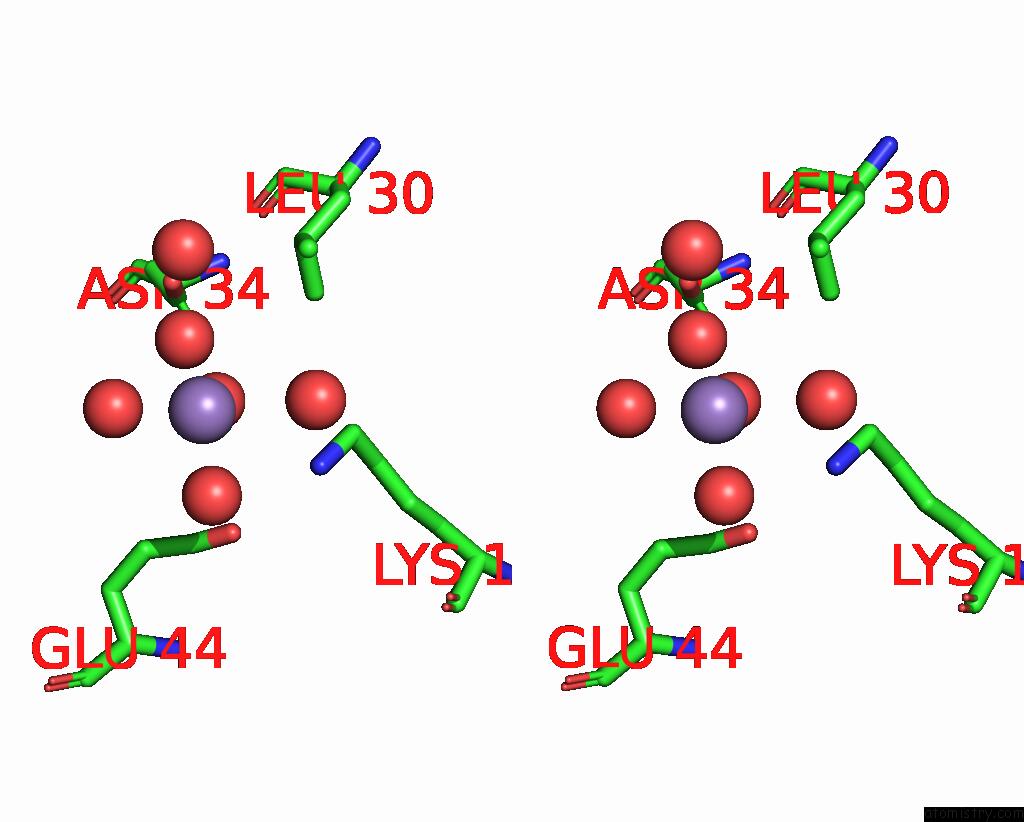
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 7 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 8 out of 10 in 1kgp
Go back to
Manganese binding site 8 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
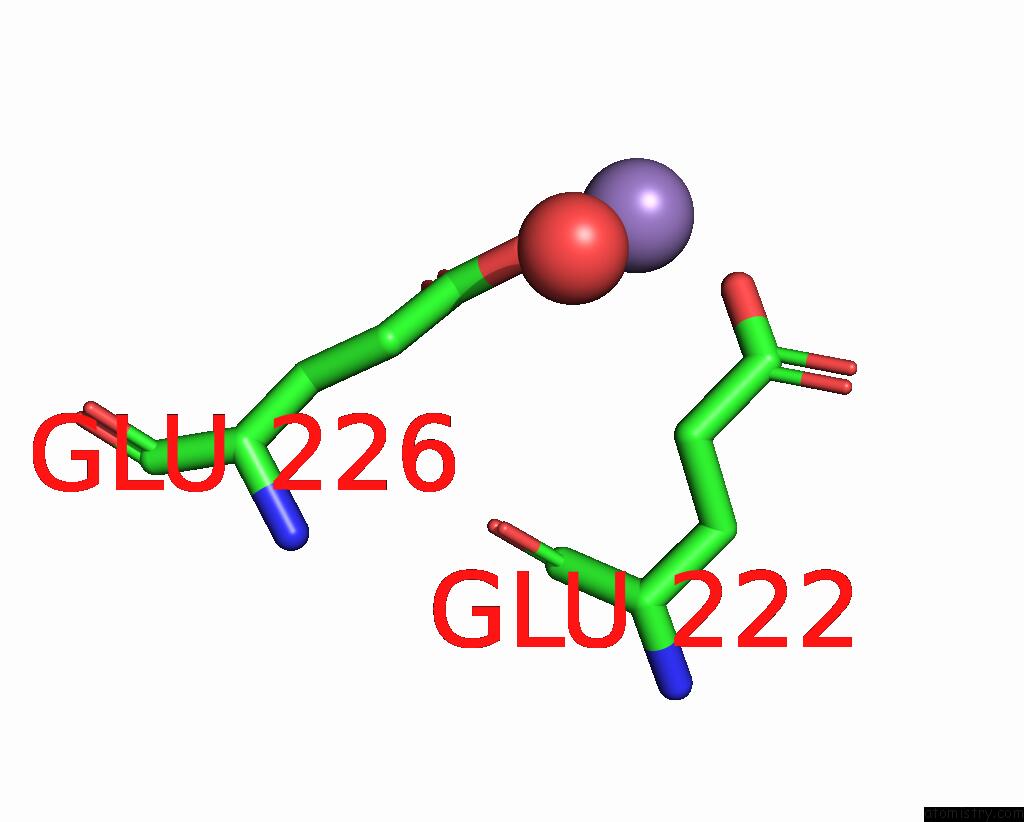
Mono view
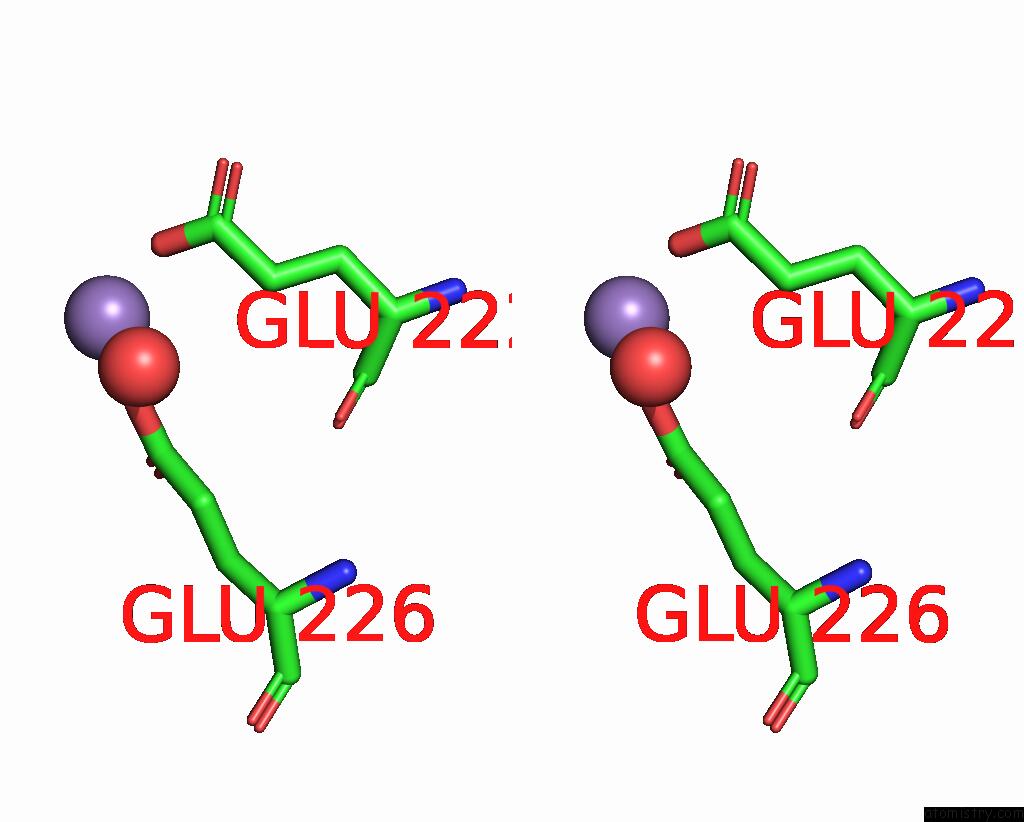
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 8 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 9 out of 10 in 1kgp
Go back to
Manganese binding site 9 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
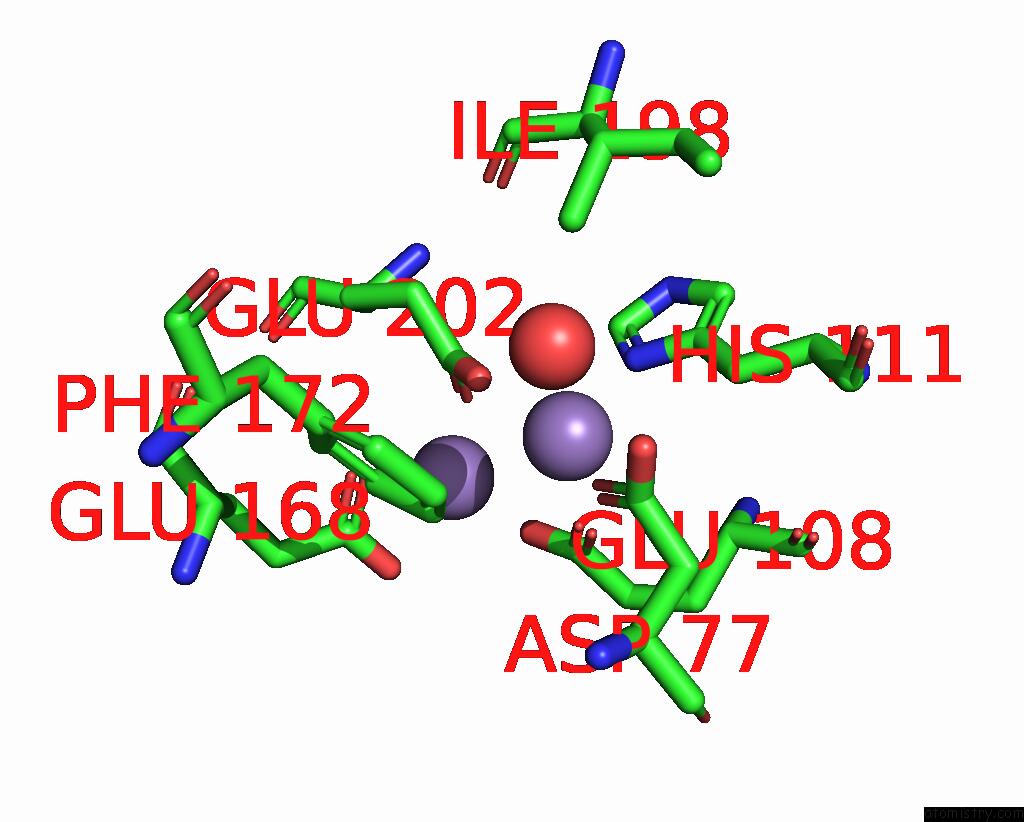
Mono view
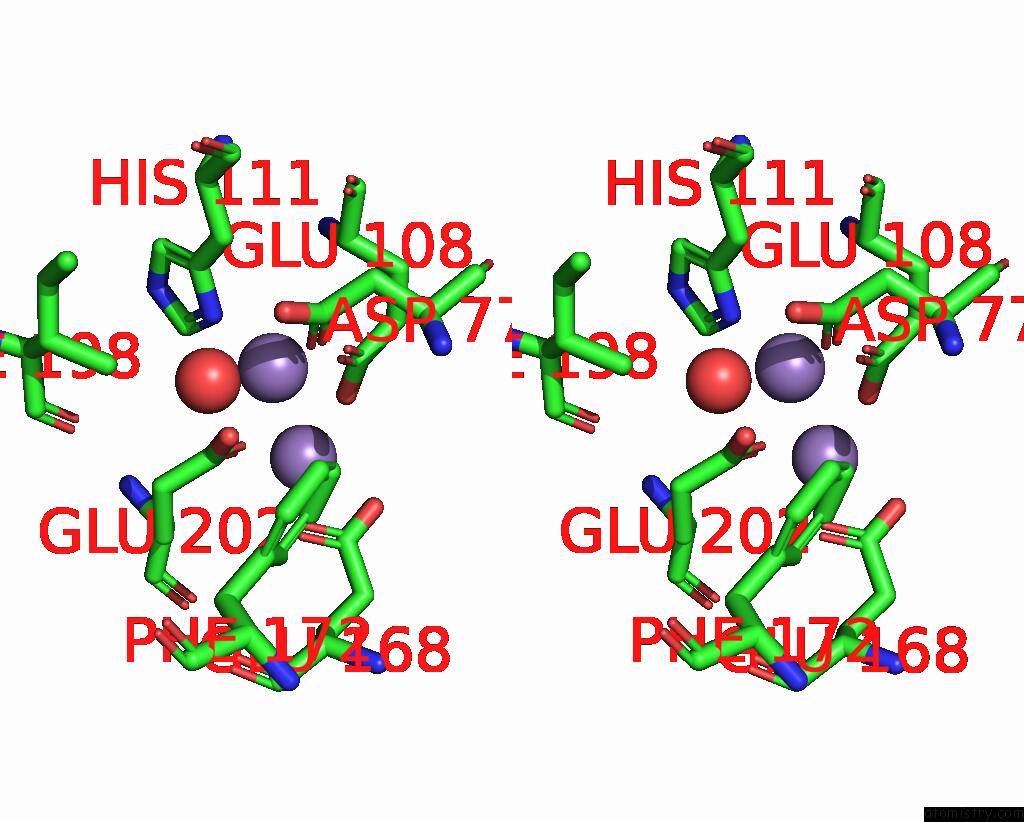
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 9 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Manganese binding site 10 out of 10 in 1kgp
Go back to
Manganese binding site 10 out
of 10 in the R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form
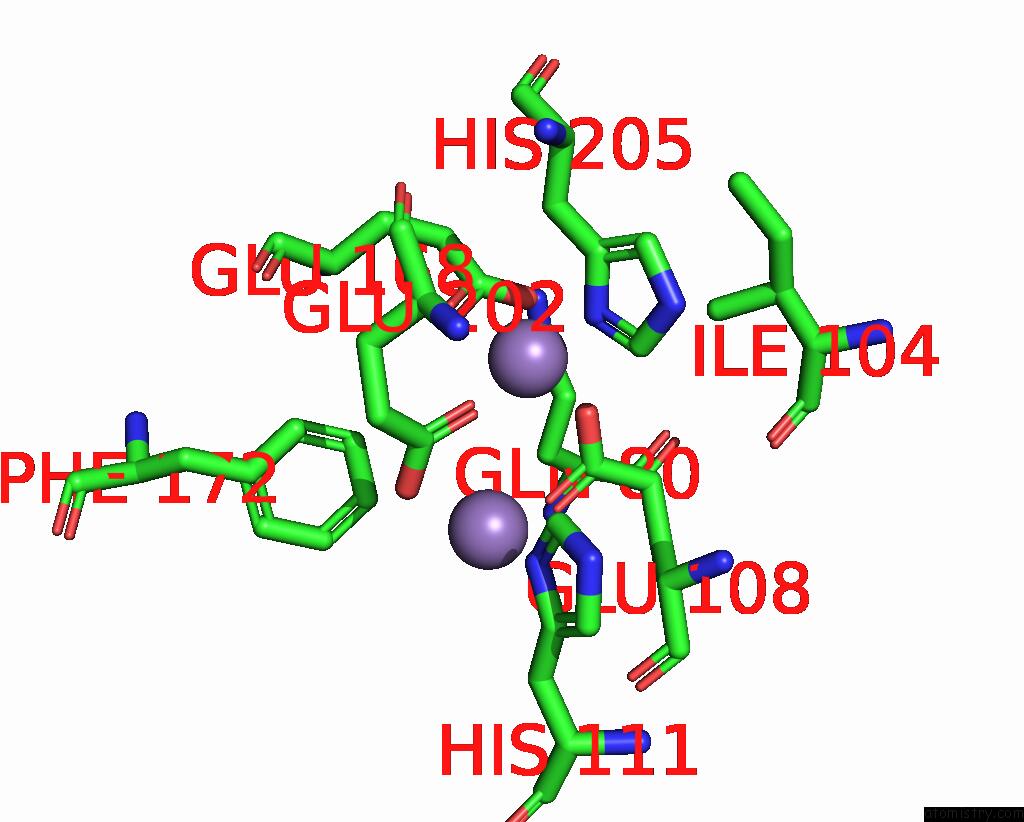
Mono view
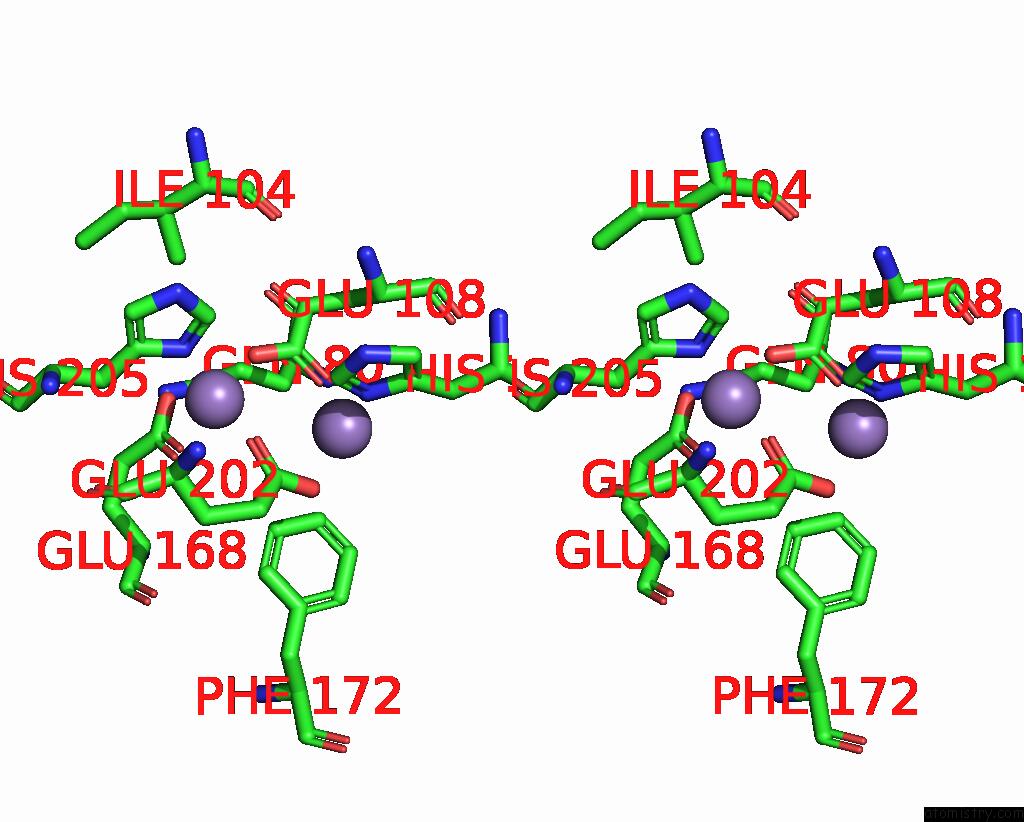
Stereo pair view

Mono view

Stereo pair view
A full contact list of Manganese with other atoms in the Mn binding
site number 10 of R2F From Corynebacterium Ammoniagenes in Its Mn Substituted Form within 5.0Å range:
|
Reference:
M.Hogbom,
Y.Huque,
B.M.Sjoberg,
P.Nordlund.
Crystal Structure of the Di-Iron/Radical Protein of Ribonucleotide Reductase From Corynebacterium Ammoniagenes. Biochemistry V. 41 1381 2002.
ISSN: ISSN 0006-2960
PubMed: 11802741
DOI: 10.1021/BI011429L
Page generated: Sat Oct 5 11:20:17 2024
ISSN: ISSN 0006-2960
PubMed: 11802741
DOI: 10.1021/BI011429L
Last articles
Ca in 2SNSCa in 2SNI
Ca in 2SNM
Ca in 2SIC
Ca in 2SEC
Ca in 2RHP
Ca in 2SAS
Ca in 2SBA
Ca in 2ROA
Ca in 2ROB